这是 达医晓护 的第 4174 篇文章
最近一段时间,全国人民正在经历奥密克戎的第一波流行高峰,是否能有治疗药物无疑是大家最为关心的事情。
据新闻报道,12月14日,中国医药将在今后一年内负责辉瑞公司的新冠病毒治疗药物Paxlovid(奈玛特韦片/利托那韦片)在中国大陆市场的进口和经销。网传有互联网平台开始销售,定价2980元一盒,但昙花一现,消息出来后当晚Paxlovid就紧急下架了。
于是朋友圈大家开始到处打听,
啥,听说这是个神药,特效,吃完后满血复活?
这是个啥药啊,那我们每个人都能吃吗?
那谁,能帮我搞一盒囤着吗?毕竟手里有药,心中不慌啊!
那要是阳了,医生能给我开这个药吗?
等等,有必要吃吗?会有啥副作用么?
今天就让我们来说说Paxlovid,你应该知道的几件事!
据小编了解,Paxloid是在今年2月12日获得国家药监局附条件批准的进口注册,目前是仅在医院内售卖的处方药。这款口服抗病毒药的主要成分是奈玛特韦和利托那韦,已纳入我国2022年《新冠肺炎治疗指南(第9版)》,见下图。

1.Paxlovid的有效率怎么样?
----目前看来,Paxlovid的有效性数据高于其他新冠药物。
根据2022年4月发表在《新英格兰医学杂志》的临床试验数据,在具有高风险进展为重症,并且未接种疫苗的轻中度新冠患者中,在出现症状后的3天内给予口服Paxlovid,相较于与口服安慰剂的对照组,可以显著降低之后28天内的住院或死亡比例89%。
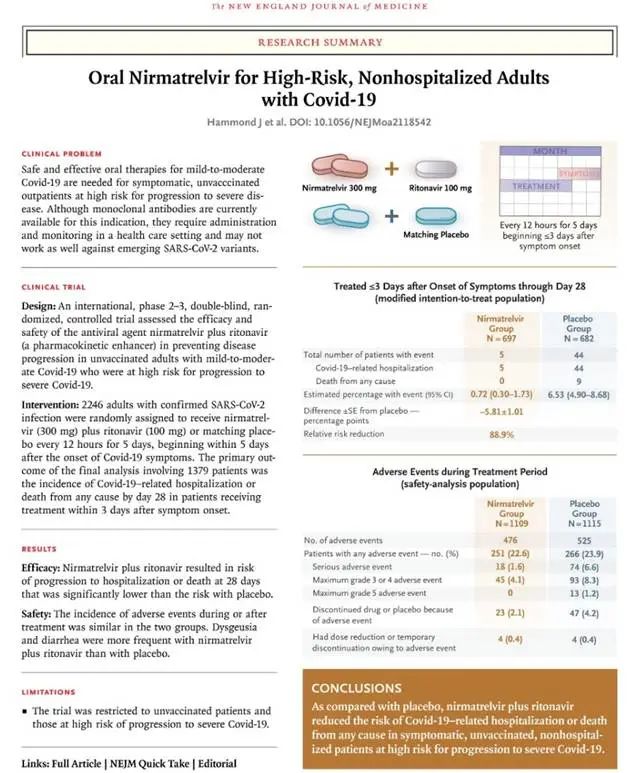
同样,对于已接种疫苗的人群研究结果也肯定了Paxlovid的有效性。2022年11月,美国疾病控制与预防中心报告了一项真实世界研究,研究纳入了接种过疫苗或以前感染过的人,在新冠确诊后5天内服用Paxlovid的成年人在之后30天内的住院率比未服用该药物的成年人低51%。

2. Paxlovid是怎么做到抗新冠病毒的?
----简而言之,二人同心,其利断金
Paxlovid是一种抗病毒的复合药物治疗,由两种单独的药物组成---奈玛特韦和利托那韦。奈玛特韦是抑制病毒复制的有效成分,可以阻断新冠病毒复制病毒颗粒过程中所需要的一种关键性的酶,这样一来,从细胞中释放的病毒就没法进入体内未感染的细胞,从而阻断感染。Paxlovid中另一个药是利托那韦,可以减缓奈玛特韦在肝脏的代谢和分解,从而提高体内奈玛特韦血药浓度和抗病毒作用。
3. Paxlovid和抗流感药达菲一样吗?
---有相同点,也有不同处
我们都知道,达菲是一种治疗季节性流感的抗病毒药物,对甲型和乙型流感病毒都有效。
这两种药物的共同点是Paxlovid和达菲都是需要在发病早期服用有效的口服抗病毒药物。达菲也是每天服用2次,持续5天,但一般希望在流感症状出现后的48小时内开始服用有效,如果超过这个时间,则不太能改变整个病程。Paxlovid也是需要在发病5天内开始服用。
但达菲的研究数据偏向于其缩短流感的病程,而Paxloid的研究重点在于它可以明显减少病人恶化进展到重症需要住院以及死亡的作用。
4. Paxlovid可以用于所有新冠阳性的病人吗?
----回答是否定的。
目前美国FDA授权Paxlovid用于12岁及以上体重至少40公斤的人群,具有新冠核酸测阳性结果,同时有进展至重症新冠感染的高风险因素, 三个条件缺一不少。比如,罹患有某些潜在疾病(包括癌症、糖尿病、肥胖症或其他疾病),或者年满65岁或65岁以上的人群,因为超过81%的新冠死亡发生在这个年龄段)。美国疾病控制与预防中心认为,个体的基础潜在疾病越多,其罹患新冠重症的风险就越高。
在我们中国,根据我国2022年《新冠肺炎治疗指南(第9版)》,重症和危重症的高危人群包括有以下人群:

综上所述,小编作为课代表总结一下, 下面这些Covid-19患者无需使用Paxlovid治疗:
1. 无症状的Covid-19感染者
2. 无进展为重症危险因素的有症状感染者
所以,我们身强力壮的中青年就不要再纠结囤药的事情了,和咱们没关系哈。
5. Paxlovid什么时候开始吃?怎么服用?
----推荐在出现症状的前五天内开始服用,越早越好。
与其他所有抗病毒药物一样,Paxlovid在发病早期,即症状出现后的前五天内效果最佳。
如下图所示,服用方法是每天2次,早晚各1次,每次口服3片(2片粉红色的奈玛特韦和1片白色的利托那韦)连续5天,整个疗程总共服用30粒。另外,Paxlovid药片应整片吞服,不能咀嚼,掰开或压碎。
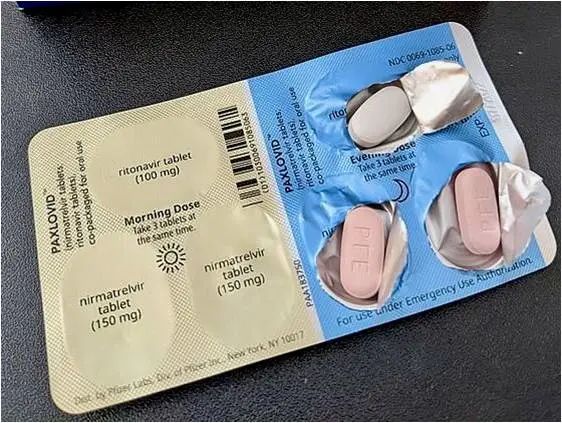
如果在应该服用的时间节点漏服一次Paxlovid,但没有超过8小时,一旦发现,可以马上补服。如果已经超过规定时间后的8小时以上,直接跳过这次服药,按原定时间服用下一剂次。不可以单次服用2剂次的药量。
6. Paxlovid有预防新冠病毒感染的作用吗?
---很遗憾,目前为止没有发现Paxlovid有预防作用。
Paxlovid并不会预防个体感染新冠病毒。目前还是主要用于治疗发病5天以内的轻型和普通型且伴有进展为重症高风险因素的成人。
7. Paxlovid有什么不良反应吗?
---对于大多数人来说Paxlovid的不良反应表现比较轻微,没有严重的副作用。
可能出现的不良反应包括有味觉改变或受损,腹泻,偶见肌肉酸痛,消化不良,呕吐,头晕,肝酶升高等。
但如果出现以下过敏反应迹象,应该停止服用Paxlovid并就医:皮疹, 吞咽或呼吸困难,口腔、嘴唇或面部肿胀, 喉部发紧,声音嘶哑。
由于Paxlovid是通过肾脏排泄,因此轻中度肾病患者需要根据医生建议调整剂量。但对于患有严重肾脏疾病、正在接受透析或患有严重肝病的患者,一般不建议使用Paxlovid,因为药物水平可能会过高,导致副作用增加。
8. Paxlovid对患者平时的常用药有影响吗?
---Paxlovid可能与多种药物发生相互作用影响疗效,医生会根据情况来指导患者暂时停药,更换药品或调整剂量。
然而,在某些情况下,药物的相互作用可能会导致严重的并发症,所以如果有正在服用禁止和Paxlovid联用的药品,医生会建议不使用Paxlovid。
下面是小编从辉瑞的Paxlovid中国版说明书中摘录的禁忌症清单,供大家参考:
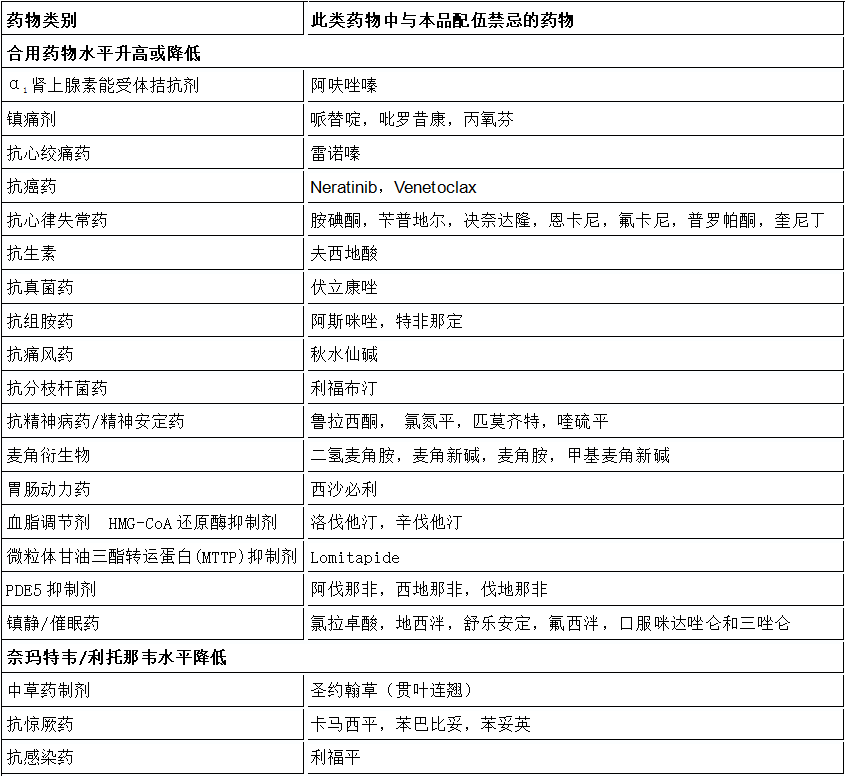
总之, 在就医时需要向医生告知目前正在使用的药物,包括处方药以及非处方药,医生会告诉患者服用Paxlovid同时使用其他药物是否安全。
9. Paxcovid国内外售价多少?
Paxloid一盒是一个疗程5天的量。在美国,一盒Paxlovid售价530美元(大约3703人民币),我国售价为2300/盒,已临时纳入医保报销。
Facts you need to know about the Paxlovid
1.How is the efficacy of the Paxlovid?
In the clinical trial published in April 2022, it had demonstrated an 89% reduction in the risk of hospitalization and death in unvaccinated people who were at high risk for proression to severe Covid-19 receiving treatment in 3 days after symptom onset.
Studies in people who have been vaccinated also confirmed the effectiveness of Paxlovid. In November 2022, the CDC reported on a real-world study that showed adults who took Paxlovid within five days of a COVID-19 diagnosis had a 51% lower hospitalization rate within the next 30 days than those who were not given the drug. The study included people who had been vaccinated or had a previous infection. The effivacy of Paxlovid is higher than other COVID-19 treatments
2.How does Paxlovid work?
Paxlovid is an antiviral therapy that consists of two separate medications packaged together--- nirmatrelvir and ritonavir. Nirmatrelvir is the key factor to fight the virus, which inhibits a key enzyme that the COVID virus requires in order to make functional virus particles. After nirmatrelvir treatment, the COVID virus that is released from the cells is no longer able to enter uninfected cells in the body, which, in turn, stops the infection. The other is ritonavir, it reduces nirmatrelvir’s metabolism in the liver.As a result, nirmatrelvir’s anti-viral works longer in the body to fight against the infection.
3.Is Paxlovid similar to Tamiflu?
As we know, Tamiflu is an antiviral drug that we use to treat seasonal flu and is effective for Flu A and Flu B.
Paxlovid and Tamiflu are both oral antiviral pills should be given early in the begining.Tamiflu is taken twice a day for five days, and it must be started within 48 hours of onset.Paxlovid is required to be started in the first 5 days of symtoms occur.
Clinical data available for Tamifly mainly focused on whether Tamiflu could shorten the length of flu illness, whearas research concentrate on preventing severe type caused hospitalization and death.
4.Can anyone who have a positive COVID-19 test get a Paxlovid?
The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. But in order to qualify for a prescription, you must also have had a positive COVID-19 test result and be at high risk for developing severe COVID-19.
That means you must either have certain underlying conditions (including cancer, diabetes, obesity, or others) or be 65 or older (more than 81% of COVID-19 deaths occur in in this group). The more underlying medical conditions a person has, the higher their risk for developing a severe case of COVID-19, according to the CDC.
5.When should I start Paxlovid? How do I take Paxlovid?
It has been recommended that Paxlovid shoule be taken within five days of developing symptoms. Like all antivirals, Paxlovid works best early in the course of an illness—in this case, within the first five days of symptom onset.
You take 2 pink nirmatrelvir tablets and 1 white ritonavir tablet together at the same time twice daily for five days for a full course that adds up to 30 pills. Swallow the tablets whole. Do not chew, break, or crush the tablets.
If you miss a dose of PAXLOVID within 8 hours of the time it is usually taken,
take it as soon as you remember. If you miss a dose by more than 8 hours, skip
the missed dose and take the next dose at your regular time. Do not take 2
doses of PAXLOVID at the same time.
6.Does Paxlovid prevent Covid-19 infection?
No. Paxlovid doesn’t prevent you from catching Covid-19. It is used to treat mild to moderate Covid-19 in people with positive viral testing and who are at high risk of progression to severe Covid-19.
7.What are the side effects from Paxlovid?
Most people who take Paxlovid should not experience serious side effects. Possible side effects include an altered or impaired sense of taste, diarrhea, increased blood pressure, muscle aches, abdominal pain, and nausea.
But people should stop taking Paxlovid and contact health care provider right away if they experience signs of an allergic reaction: skin rash, trouble swallowing or breathing
swelling of the mouth, lips, or face, throat tightness, hoarseness.
Since Paxlovid is cleared by the kidneys, dose adjustments may be required for patients with mild-to-moderate kidney disease. For patients with severe kidney disease—or who are on dialysis—or those with severe liver disease, Paxlovid is not recommended; the levels of the drug can become too high and could cause increased side effects.
8.Does Paxlovid interact with other medications I am taking?
There is a long list of medications Paxlovid may interact with and doctors will give advice to patient whether there is a need to temporarily discontinue the medications, change the medication, or regulate the dosage. While in some cases, doctors may not prescribe Paxlovid because these interactions may cause serious complications. The following lists are the contraindications from Pfizer Paxlovid fact sheet.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Your doctor can tell you if it is safe to take Paxlovid with other medicines.
PAXLOVID is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions :
·Alpha1-adrenoreceptor antagonist: alfuzosin
·Antianginal: ranolazine
·Antiarrhythmic: amiodarone, dronedarone, flecainide, propafenone, quinidine
·Anti-gout: colchicine
·Antipsychotics: lurasidone, pimozide
·Benign prostatic hyperplasia agents: silodosin
·Cardiovascular agents: eplerenone, ivabradine
·Ergot derivatives: dihydroergotamine, ergotamine, methylergonovine
·HMG-CoA reductase inhibitors: lovastatin, simvastatin
·Immunosuppressants: voclosporin
·Microsomal triglyceride transfer protein inhibitor: lomitapide
·Migraine medications: eletriptan, ubrogepant
·Mineralocorticoid receptor antagonists: finerenone
·Opioid antagonists: naloxegol
·PDE5 inhibitor: sildenafil (Revatio®) when used for pulmonary arterial hypertension (PAH)
·Sedative/hypnotics: triazolam, oral midazolam
·Serotonin receptor 1A agonist/serotonin receptor 2A antagonist: flibanserin
·Vasopressin receptor antagonists: tolvaptan
PAXLOVID is contraindicated with drugs that are potent CYP3A inducers where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance. PAXLOVID cannot be started immediately after discontinuation of any of the following medications due to the delayed offset of the recently discontinued CYP3A inducer:
·Anticancer drugs: apalutamide
·Anticonvulsant: carbamazepine, phenobarbital, primidone, phenytoin
·Cystic fibrosis transmembrane conductance regulator potentiators: lumacaftor/ivacaftor
·Antimycobacterials: rifampin
·Herbal products: St. John's Wort (hypericum perforatum)
参考文献:
1.中华人民共和国国家卫生健康委员会办公厅, 中华人民共和国国家中医药管理局办公室.新型冠状病毒肺炎诊疗方案(试行第九版)[EB/OL].(2022-03-15). http://www.nhc.gov.cn/cms-search/downFiles/ef09aa4070244620b010951b088b8a27.pdf
2.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022; 386:1397-1408. DOI: 10.1056/NEJMoa2118542
3.Shah MM, Joyce B, Plumb ID, et al. Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 — United States, April–September 2022. MMWR Morb Mortal Wkly Rep 2022;71:1531–1537. DOI: http://dx.doi.org/10.15585/mmwr.mm7148e2.
4.https://www.pfizermedicalinformation.cn/products/package-insert/paxlovid
5.https://www.yalemedicine.org/news/13-things-to-know-paxlovid-covid-19
6.https://labeling.pfizer.com/ShowLabeling.aspx?id=16670
7.https://labeling.pfizer.com/ShowLabeling.aspx?id=16474
作者:澳大利亚墨尔本大学医学院儿科博士
百汇医疗(中国)儿科医师
蒋本然







 扫码下载APP
扫码下载APP

 科普中国APP
科普中国APP
 科普中国
科普中国
 科普中国
科普中国