摘要
Abstract
美国临床肿瘤学会(ASCO)指南提供了每项建议的全面文献审查和分析,并遵循ASCO指南方法手册中概述的指南制定流程。ASCO指南遵循ASCO临床实践指南利益冲突政策。
ASCO Guidelines provide recommendations with comprehensive review and analyses of the relevant literature for each recommendation, following the guideline development process as outlined in the ASCO Guidelines Methodology Manual. ASCO Guidelines follow the ASCO Conflict of Interest Policy for Clinical Practice Guidelines.
ASCO提供的临床实践指南和其他指导(“指导”)并非治疗选择的全面或决定性指南。它旨在供提供者自愿使用,并应与独立的专业判断结合使用。指导可能不适用于所有患者、干预措施、疾病或疾病阶段。指导基于相关文献的审查和分析,不旨在作为标准护理的声明。ASCO不支持第三方药物、设备、服务或疗法,并对使用此信息可能引起的任何损害不承担任何责任。有关更多信息,请参见附录1和附录2(仅限在线)中的完整免责声明。
Clinical Practice Guidelines and other guidance (“Guidance”) provided by ASCO is not a comprehensive or definitive guide to treatment options. It is intended for voluntary use by providers and should be used in conjunction with independent professional judgment. Guidance may not be applicable to all patients, interventions, diseases or stages of diseases. Guidance is based on review and analysis of relevant literature, and is not intended as a statement of the standard of care. ASCO does not endorse third-party drugs, devices, services, or therapies and assumes no responsibility for any harm arising from or related to the use of this information. See complete disclaimer in Appendix 1 and Appendix 2 (online only) for more.
目的
Purpose
指导实体瘤或造血系统恶性肿瘤成年患者的疫苗接种。
To guide the vaccination of adults with solid tumors or hematologic malignancies.
方法
Methods
系统性文献回顾识别了有关成年癌症患者或其家庭成员使用的疫苗的有效性和安全性的系统评价、随机对照试验(RCTs)和非随机研究。这项回顾基于2013年美国传染病学会的指南。从2013年1月1日到2023年2月16日,检索了PubMed和Cochrane图书馆。ASCO召集了一个专家小组来审查证据并制定建议。
A systematic literature review identified systematic reviews, randomized controlled trials (RCTs), and nonrandomized studies on the efficacy and safety of vaccines used by adults with cancer or their household contacts. This review builds on a 2013 guideline by the Infectious Disease Society of America. PubMed and the Cochrane Library were searched from January 1, 2013, to February 16, 2023. ASCO convened an Expert Panel to review the evidence and formulate recommendations.
结果
Results
系统评价共纳入102篇出版物:24篇系统评价、14项随机对照试验和64项非随机研究。最大的证据体系涉及新冠疫苗。
A total of 102 publications were included in the systematic review: 24 systematic reviews, 14 RCTs, and 64 nonrandomized studies. The largest body of evidence addressed COVID-19 vaccines.
建议
Recommendations
疫苗接种的目标是限制感染的严重程度并在可行的情况下预防感染。优化疫苗接种状态应被视为癌症患者护理的关键要素。这种方法包括在首次患者就诊时记录疫苗接种状态;及时提供推荐的疫苗;以及在造血干细胞移植、嵌合抗原受体T细胞疗法或B细胞耗竭疗法后适当地重新接种疫苗。医疗保健提供者之间,包括初级保健医生、药剂师和护理团队成员,需要积极的互动和协调。为癌症患者的家庭成员接种疫苗将增强对患者的保护。癌症患者的一些疫苗接种和重新接种计划可能受到患者基础免疫状态和接受的抗癌治疗的影响。因此,疫苗策略可能与一般健康成人人群疫苗的推荐不同。
The goal of vaccination is to limit the severity of infection and prevent infection where feasible. Optimizing vaccination status should be considered a key element in the care of patients with cancer. This approach includes the documentation of vaccination status at the time of the first patient visit; timely provision of recommended vaccines; and appropriate revaccination after hematopoietic stem-cell transplantation, chimeric antigen receptor T-cell therapy, or B-cell–depleting therapy. Active interaction and coordination among healthcare providers, including primary care practitioners, pharmacists, and nursing team members, are needed. Vaccination of household contacts will enhance protection for patients with cancer. Some vaccination and revaccination plans for patients with cancer may be affected by the underlying immune status and the anticancer therapy received. As a result, vaccine strategies may differ from the vaccine recommendations for the general healthy adult population vaccine.
更多信息可在www.asco.org/supportive-care-guidelines上获得。
Additional information is available at www.asco.org/supportive-care-guidelines.
引言
Introduction
由于包括慢性炎症、造血系细胞元素功能受损和/或减少,以及损害免疫功能的治疗在内的多种因素,癌症患者常常经历免疫系统受损。1-4 因此,癌症患者面临较高的感染风险,这种风险可能超出癌症治疗范围,突显了肿瘤学家需要与初级保健提供者合作,作为标准肿瘤学评估的一部分,获取最新的疫苗接种历史,并解决预防疫苗可预防疾病的干预措施的需求。疫苗对癌症患者的感染防护效果与免疫抑制的程度和类型以及潜在恶性肿瘤的严重程度相关。5-9 疫苗接种的目的是保护免受感染并减轻无法完全预防的感染的病程严重程度。
Patients with cancer often experience a compromised immune system because of a variety of factors, including chronic inflammation, impaired and/or decreased function of elements of the hematopoietic lineage, and treatments that compromise immune function.1-4 Consequently, patients with cancer are at a heightened risk for infection, which can extend beyond cancer treatment, highlighting the need for oncologists to partner with primary care providers to obtain an up-to-date vaccine history as part of the standard oncologic evaluation and to address intervention for vaccine-preventable diseases. The efficacy of vaccines against infection in patients with cancer correlates with the degree and type of immunosuppression and/or severity of underlying malignancy.5-9 The purpose of vaccination is to protect from infection and attenuate the severity of disease where infection cannot be fully prevented.
目标人群和受众
Target Population and Audience
目标人群
Target Population
患有实体瘤或造血系统恶性肿瘤的成年人,包括接受造血干细胞移植、嵌合抗原受体T细胞疗法和B细胞耗竭疗法的患者,以及长期存活者及其家庭成员。
Adults with solid tumors or hematologic malignancies, including those who receive hematopoietic stem-cell transplantation, chimeric antigen receptor T-cell therapy and B-cell–depleting therapies, and long-term survivors, and their household contacts.
目标受众
Target Audience
癌症患者以及在癌症治疗前、中、后为他们提供护理的临床医生。
Adults with cancer and the clinicians who provide care to them before, during, and after cancer treatment.
在临床实践中存在的固有变异性,包括一些癌症患者缺乏初级保健医生,强化了需要一种适用于为医疗复杂的肿瘤患者群体提供护理的医疗团队的疫苗接种方法的需求。活病毒疫苗通常不适用于免疫系统严重受损的患者。5,10 相反,非活疫苗通常被认为是安全的,但它们产生免疫反应的能力可能根据免疫抑制的净状态而有所不同。5,11,12
Inherent variability in clinical practice, including the lack of primary care physicians for some patients with cancer, reinforces the need for an approach to vaccination applicable to the health care teams caring for the medically complex oncology population. Live virus vaccines are typically contraindicated in patients with severely compromised immune systems.5,10 Conversely, nonlive vaccines are generally considered safe, but their ability to produce immune responses may differ depending on the net state of immunosuppression.5,11,12
为了提高癌症患者的疫苗接种率,ASCO已与美国疾病控制与预防中心(CDC)、医学专业学会委员会和其他专业学会合作,达成了为期5年的合作协议。13 除了支持本指南的制定外,合作协议还包括提供者教育、患者教育和质量改进的工作。ASCO已与八个美国卫生系统合作,这些系统正在按照成人免疫实践标准中概述的流程改进符合指南的疫苗接种。14
To improve vaccination rates among patients with cancer, ASCO has engaged with the Centers for Disease Control and Prevention (CDC), Council of Medical Specialty Societies, and other specialty societies in a 5-year cooperative agreement.13 Along with supporting the development of this guideline, the cooperative agreement includes efforts in provider education, patient education, and quality improvement. ASCO has engaged with eight US health systems that are improving guideline-concordant vaccination following processes outlined in the Standards for Adult Immunization Practice.14
本ASCO指南组织了癌症患者推荐的疫苗,并确定了需要重新接种疫苗的独特环境以及该过程的时机。指南建议在表1中提供。疫苗大致分为活疫苗和非活疫苗,以遵循标准命名法,该命名法区分了对正在接受癌症治疗的个体安全的疫苗和应避免的疫苗。
This ASCO guideline organizes recommended vaccines for patients with cancer and identifies unique settings where revaccination is needed and the timing for that process. Guideline recommendations are provided in Table 1. Vaccines are broadly categorized into live and nonlive vaccines to follow a standard nomenclature that identifies vaccines that are safe for individuals undergoing cancer treatment and those that should be avoided.
活疫苗:减毒活疫苗含有减毒但仍能复制的病毒或细菌。它们通过在健康个体中引起低度感染来诱导持久免疫。然而,在免疫系统受损的患者中,活疫苗可能会导致来自疫苗株的无法控制的感染,因此应避免。活疫苗的例子包括水痘疫苗、麻疹和腮腺炎及风疹联合疫苗(MMR)以及口服伤寒疫苗。
Live vaccines: Live-attenuated vaccines contain an attenuated but replicating virus or bacteria. They induce durable immunity by causing a low-grade infection in otherwise healthy individuals. However, in patients with weakened immune systems, live vaccines can pose a risk of uncontrolled infection from the vaccine strain and are therefore avoided. Examples of live vaccines include varicella; the measles, mumps, and rubella (MMR) vaccine; and oral typhoid.
非活疫苗:这些疫苗对癌症患者是安全的。目前可用的非活疫苗包括灭活疫苗;亚单位疫苗,包括重组、多糖疫苗和多糖-蛋白结合疫苗;类毒素疫苗;以及mRNA疫苗。
Nonlive vaccines: These vaccines are safe for use in patients with cancer. Currently available nonlive vaccines include inactivated vaccines; subunit vaccines, including recombinant, polysaccharide vaccines and polysaccharide-protein conjugate vaccines; toxoids; and mRNA vaccines.
表1:整体建议摘要
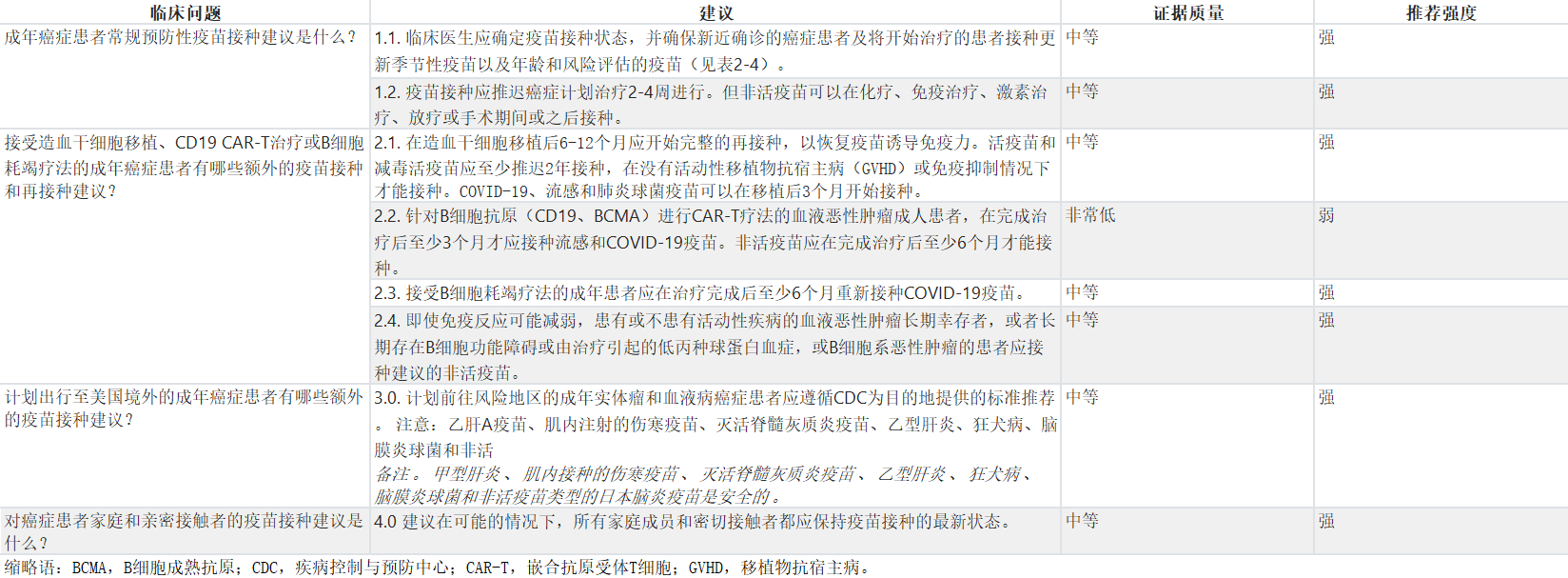
Table 1. Summary of All Recommendations
为了给癌症患者提供关于不同情况下疫苗类型和接种时间的建议,考虑了与实际相关的问题,包括固体肿瘤患者、血液恶性肿瘤患者、接受移植和嵌合抗原受体T细胞(CAR-T)疗法的患者以及长期幸存者的免疫接种细节,以及对免疫功能受损宿主的疫苗接种时间表进行了整理。本指南将参考CDC的免疫接种实践咨询委员会(ACIP)目前建议的疫苗接种时间表,并确定在肿瘤学人群中是否建议进行变更,以及何时进行变更以及变更内容。癌症患者同时存在其他医疗条件的情况下,可能还可以接种额外的疫苗。这些疫苗以及应考虑或避免的特定风险情况在表3中显示。最后,表4显示了对于可能在儿童时期未曾接种全部或部分常规疫苗的癌症患者建议接种的疫苗。本指南不涉及19岁以下患者的疫苗接种建议,也不为同时患有HIV和癌症的患者指定特殊的建议。
Practical questions about vaccines in patients with cancer were considered to provide recommendations on types and timing of vaccines for different scenarios faced by patients, including the nuances of immunizations in patients with solid tumors, hematologic malignancies, patients receiving transplant and chimeric antigen receptor T-cell (CAR-T) therapy, and unique needs for long-term survivors. Table 2 collates the currently advised vaccination schedule from the CDC's Advisory Committee on Immunization Practices (ACIP) for immunocompromised hosts. This guideline will refer to this table and identify if, when, and what changes are recommended for an oncology population. Patients with cancer and coexisting medical conditions may also be eligible to receive additional vaccines. These vaccines and the specific risk situations where they should be considered or avoided are shown in Table 3. Finally, Table 4 shows the recommended vaccinations for patients with cancer who might not have received all or some of the routinely recommended immunizations as children. This guideline does not address vaccination recommendations for patients younger than 19 years, nor does it specify unique recommendations for patients living with HIV who also have cancer.
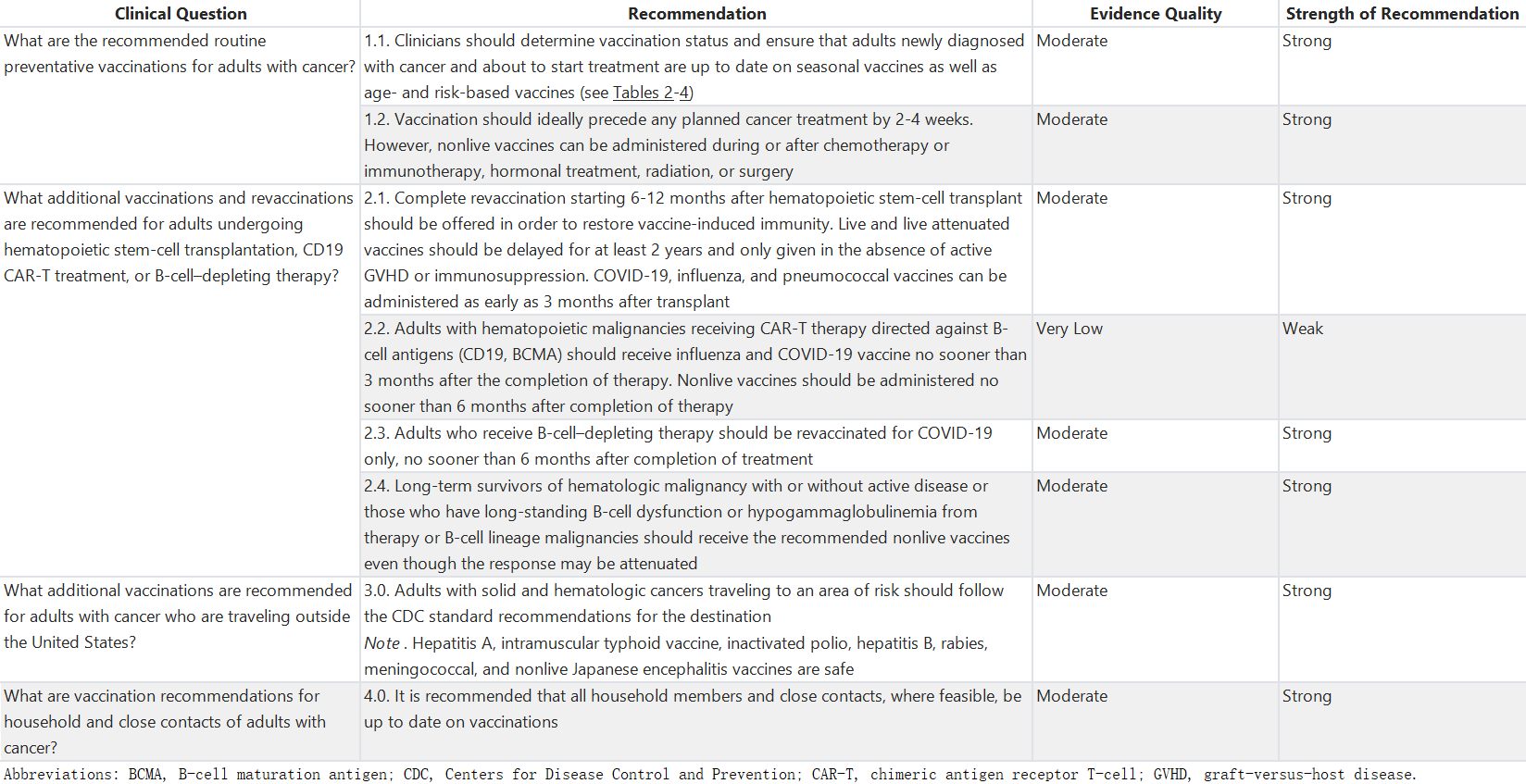
表2:建议成年癌症患者接种的疫苗
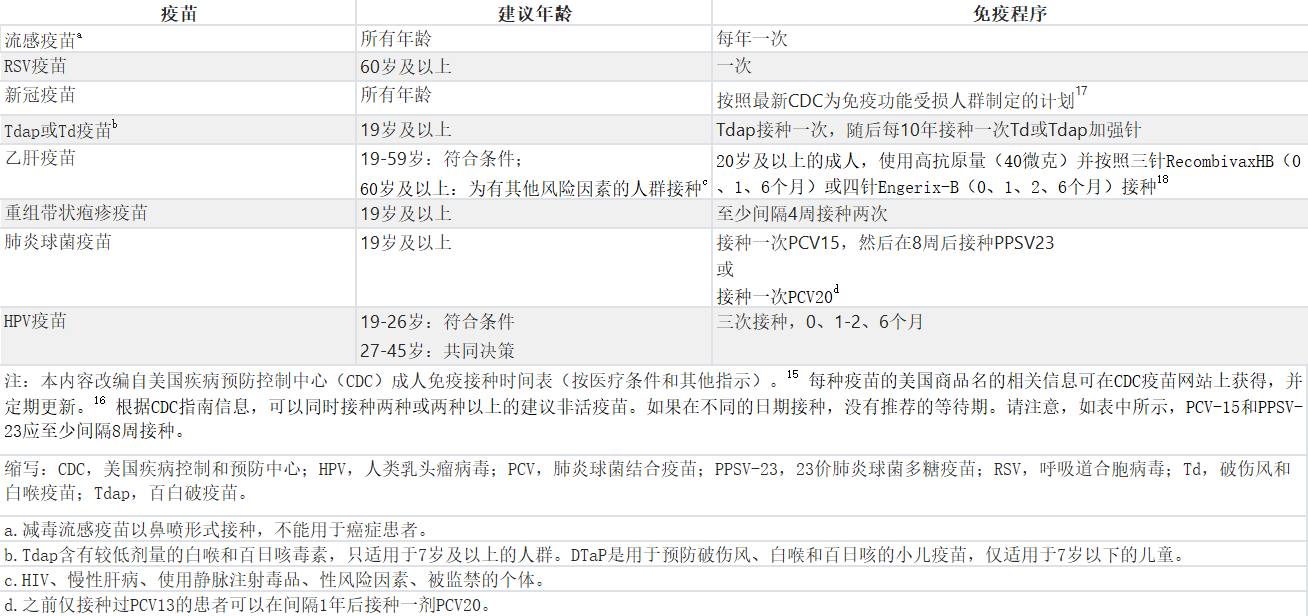
Table 2. Recommended Immunizations for Adults With Cancer
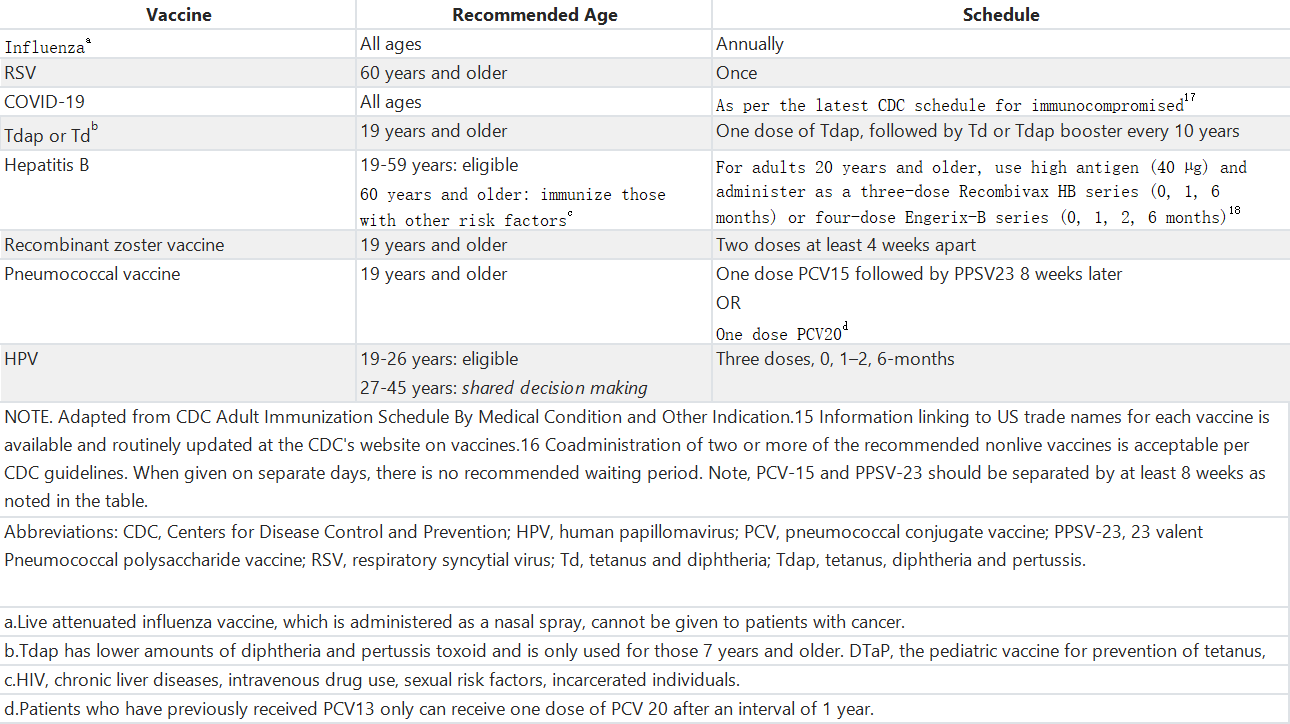
表3. 对于患有癌症及合并健康状况的成人可能适用的其他疫苗建议
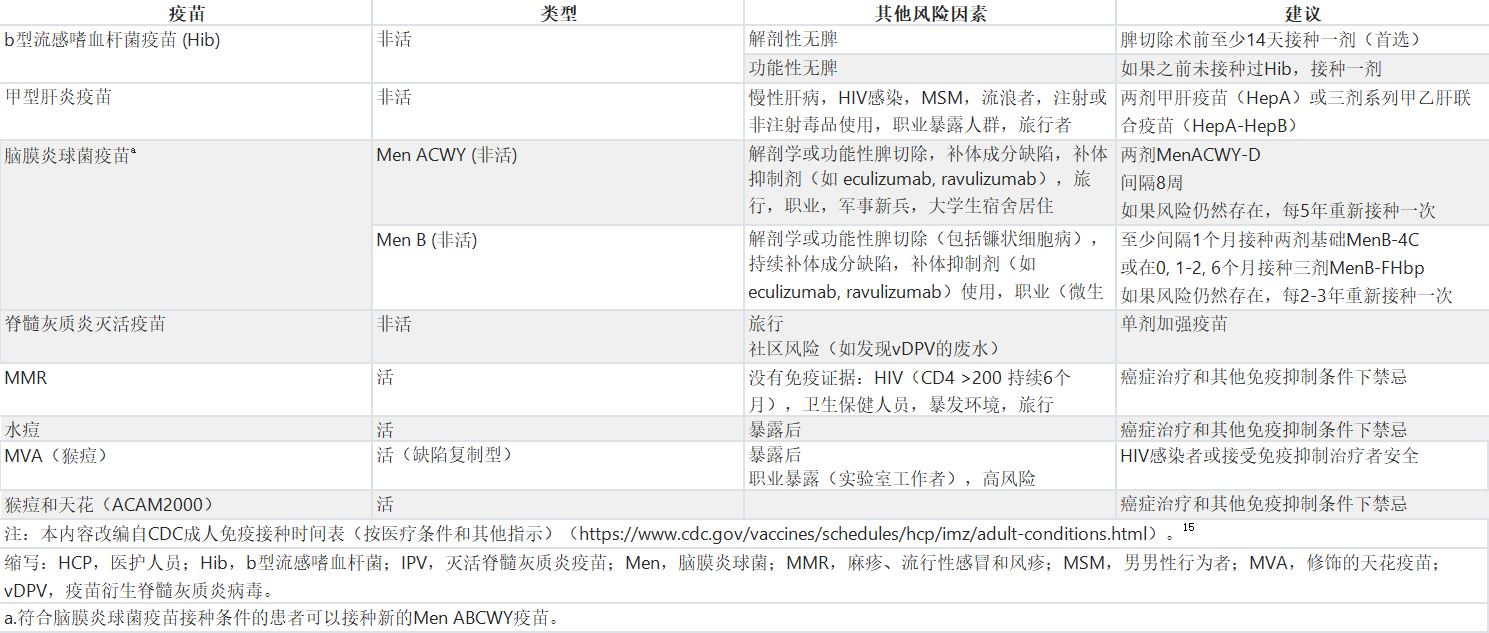
Table 3. Recommendations for Other Vaccines That May be Indicated for Adults With Cancer and Coexisting Health Conditions
指南问题
Guideline Questions
该临床实践指南涉及四个主要的临床问题:(1) 对于患有癌症的成年人,推荐的常规预防性疫苗是什么?(2) 对于接受造血干细胞移植(HSCT)、CD19 CAR-T治疗或B细胞耗竭疗法的成年人,推荐哪些额外接种和再接种?(3) 对于计划到美国以外地区旅行的癌症成年人,推荐哪些额外接种?(4) 对于癌症成年患者的家庭成员和密切接触者,推荐哪些疫苗接种?
This clinical practice guideline addresses four overarching clinical questions: (1) What are the recommended routine preventative vaccinations for adults with cancer? (2) What additional vaccinations and revaccinations are recommended for adults undergoing hematopoietic stem-cell transplantation (HSCT), CD19 CAR-T treatment, or B-cell–depleting therapy? (3) What additional vaccinations are recommended for adults with cancer who are traveling outside the United States? (4) What are vaccination recommendations for household and close contacts of adults with cancer?
方法
Methods
指南制定流程
Guideline Development Process
该基于系统评价的指南是由一组志愿者、多学科的专家小组制定的,其中包括一个患者代表和一个具有健康研究方法学专业知识的ASCO指南工作人员(附录表A1)。
This systematic review-based guideline was developed by a volunteer, multidisciplinary Expert Panel, which included a patient representative and an ASCO guidelines staff member with health research methodology expertise (Appendix Table A1).
推荐建议是在从2013年1月1日到2023年2月16日通过对PubMed和Cochrane图书馆进行在线搜索获得,并通过系统回顾证据进行制定的。这个系统回顾基于2013年美国传染病学会(IDSA)关于免疫功能受损宿主疫苗接种的指南5,并可能引用先前提供推荐证据基础且这些没有发表更新版本的文献。符合以下标准的文章被选入系统回顾:
The recommendations were developed using a systematic review of evidence identified through online searches of PubMed and the Cochrane Library from January 1, 2013, to February 16, 2023. This systematic review builds upon the 2013 Infectious Disease Society of America (IDSA) guideline on Vaccination of the Immunocompromised Host5 and may cite previous literature that provides the evidence base for the recommendation and for which no updates have been published. Eligible publication types were systematic reviews, randomized controlled trials (RCTs), and nonrandomized studies (for questions not addressed by systematic reviews or RCTs). Articles were selected for inclusion in the systematic review on the basis of the following criteria:
受试者:实体肿瘤或血液恶性肿瘤的成年患者,包括长期幸存者和接受HSCT的患者。
Population: Adults with solid tumors or hematologic malignancies, including long-term survivors and those who received HSCT.
干预措施:CDC免疫时间表和旅行疫苗中列出的常规疫苗。
Interventions: Routine vaccines listed on the CDC immunization schedules and travel vaccines.
对照:安慰剂,不同疫苗,或不同疫苗接种策略(例如不同的接种时间)。
Comparisons: Placebo, different vaccines, or different strategies for vaccination (eg, different vaccine timings).
结果:感染风险、疾病严重程度、死亡率、细胞和体液免疫应答以及疫苗安全性。
Outcomes: Risk of infection, disease severity, mortality, cellular and humoral immune responses, and vaccine safety.
被排除出系统回顾的文章包括(1)未随后发表在同行评审期刊上的会议摘要;(2)社论、评论、来信、新闻文章、病例报告和叙述性综述;以及(3)非英语发表的文章。
Articles were excluded from the systematic review if they were (1) meeting abstracts not subsequently published in peer-reviewed journals; (2) editorials, commentaries, letters, news articles, case reports, and narrative reviews; and (3) published in a non-English language.
共举行了三次全体专家小组会议,成员被要求在指南制定过程中持续提供对制定方案、证据质量和评估、推荐生成的意见;起草内容;以及在整个指南制定过程中审查和批准草稿。。ASCO工作人员定期与专家小组共同主席会面,并通过电子邮件与小组进行协调沟通,以完成整个制定过程。每项建议都附有推荐强度和证据质量的评级,定义见附录表A2。使用Cochrane偏倚风险工具和GRADE质量评估和推荐开发过程的要素评估了每个结果的证据质量。19,20 项目方法学家与专家小组共同主席合作,为每个结果分配GRADE质量评估标签(即高、中、低、非常低),并由全体专家小组审查。
Three full panel meetings were held, and members were asked to provide ongoing input on the guideline development protocol, quality and assessment of the evidence, and generation of recommendations; draft content; and review and approve drafts during the entire development of the guideline. ASCO staff met routinely with the Expert Panel cochairs and corresponded with the panel via e-mail to coordinate the process to completion. Ratings for the strength of the recommendation and evidence quality are provided with each recommendation, defined in Appendix Table A2. The quality of the evidence for each outcome was assessed using the Cochrane Risk of Bias tool and elements of the GRADE quality assessment and recommendation development process.19,20 GRADE quality assessment labels (ie, high, moderate, low, very low) were assigned for each outcome by the project methodologist in collaboration with the Expert Panel cochairs and reviewed by the full Expert Panel.
指南审查和批准
Guideline Review and Approval
初稿建议从2023年7月28日至8月11日期间向公众开放评论。对于每条建议,记录了“同意原文”、“同意并提出修改建议”和“不同意。见评论”等回应类别,共收到47条书面评论。针对每条建议,同意或同意稍作修改的受访者比例在88%至100%之间。专家小组成员审查了来自所有来源的评论,并决定是保留原始草案建议、进行轻微语言修改、还是考虑进行重大建议修订。此外,进行了指南可实施性审查。根据这一审查结果,对初稿进行了修改,以澄清临床实践的推荐行动。
The draft recommendations were released to the public for open comment from July 28 to August 11, 2023. Response categories of “Agree as written,” “Agree with suggested modifications,” and “Disagree. See comments” were captured for every proposed recommendation, with 47 written comments received. For each recommendation, the proportion of respondents who agreed or agreed with slight modifications ranged from 88% to 100%. Expert Panel members reviewed comments from all sources and determined whether to maintain the original draft recommendations, revise with minor language changes, or consider major recommendation revisions. In addition, a guideline implementability review was conducted. On the basis of this review, revisions were made to the draft to clarify the recommended actions for clinical practice.
所有修改均在最终手稿提交给ASCO循证医学委员会(EBMC)审查和批准之前进行。所有ASCO指南最终在提交给《临床肿瘤学杂志》进行编辑审查和考虑发表之前,都将经过专家小组和ASCO EBMC的审查和批准。
All changes were incorporated into the final manuscript before ASCO Evidence-Based Medicine Committee (EBMC) review and approval. All ASCO guidelines are ultimately reviewed and approved by the Expert Panel and the ASCO EBMC before submission to the Journal of Clinical Oncology for editorial review and consideration for publication.
指南更新
Guideline Updating
ASCO专家小组和指南工作人员将与共同主席合作,及时了解指南的任何实质性更新。根据对新发布文献的正式审查,ASCO将确定更新的需求。ASCO指南方法手册(可在www.asco.org/guideline-methodology获取)提供了有关指南更新过程的额外信息。这是截至出版日期的最新信息。
The ASCO Expert Panel and guideline staff will work with cochairs to keep abreast of any substantive updates to the guideline. On the basis of a formal review of the emerging literature, ASCO will determine the need to update. The ASCO Guidelines Methodology Manual (available at www.asco.org/guideline-methodology) provides additional information about the guideline update process. This is the most recent information as of the publication date.
结果
Results
在文献检索中发现的研究特征
Characteristics of Studies Identified in the Literature Search
共获得了1,596篇文献。经过应用资格标准筛选后剩余102项研究作为指南建议的证据基础,其中包括:24项系统回顾,11,21-43 14项随机对照试验,6,44-56 和64项非随机研究。57-84,85-104,105-120
A total of 1,596 publications were identified in the literature search. After applying the eligibility criteria, 102 studies remained, forming the evidentiary basis for the guideline recommendations: 24 systematic reviews,11,21-43 14 RCTs,6,44-56 and 64 nonrandomized studies.57-84,85-104,105-120
这些研究的发表时间横跨2013年至2023年。主要研究集中在新冠疫苗的免疫原性,以及这可能因癌症类型和治疗方式而异。非随机研究还评估了新冠疫苗接种状况与癌症患者COVID-19感染和住院情况之间的关系。对于流感疫苗,随机对照试验考虑了接种时间、疫苗类型或剂次对免疫原性或流感结果的影响。对于COVID-19和流感疫苗,系统回顾探讨了疫苗接种是否会影响免疫相关不良事件的频率。较少的研究涉及其他常规成人疫苗。系统回顾和随机对照试验的特征和结果详见数据补充。非随机研究按主题列在补充中,但未提取完整数据。
The identified trials were published between 2013 and 2023. The largest body of evidence focused on the immunogenicity of COVID-19 vaccines and how this may vary by type of cancer and cancer therapy. Nonrandomized studies have also evaluated the association between COVID-19 vaccination status and COVID-19 infections and hospitalizations in patients with cancer. For influenza vaccines, RCTs considered whether variations in the timing of administration, vaccine type, or number of doses affect immunogenicity or influenza outcomes. For both COVID-19 and influenza vaccines, systematic reviews have explored whether vaccination affects the frequency of immune-related adverse events. Smaller numbers of studies addressed other routine adult vaccines. Characteristics and results of the systematic reviews and RCTs are provided in the Data Supplement. Nonrandomized studies are listed by topic in the Supplement, but data were not extracted in full.
证据质量评估
Evidence Quality Assessment
针对每个感兴趣的结果评估了证据质量。这个评级包括研究设计、结果的一致性、证据的直接性、精确性、出版偏倚以及效果的大小等因素,由一名评审员评估。除了CAR-T疗法后疫苗接种的最佳时间方面几乎没有证据外,其余情况下的证据质量评级均为中等。对于其他情况的中等评级,是由于在癌症患者中随机对照试验相对较少,但来自非随机研究的一致证据体系。有关证据质量的定义,请参考附录表A2,更多信息请参阅方法手册。
The quality of evidence was assessed for each outcome of interest. This rating includes factors such as study design, consistency of results, directness of evidence, precision, publication bias, and magnitude of effect, assessed by one reviewer. Evidence quality was rated as moderate with one exception: very little evidence is available regarding the optimal timing of vaccination after CAR-T therapy. The moderate rating for remaining settings was due to the relatively small number of RCTs in patients with cancer, but a consistent body of evidence from nonrandomized studies. Refer to Appendix Table A2 for definitions of the quality of the evidence, and the Methodology Manual for more information.
建议
Recommendations
所有建议均可在表1中获得。
All recommendations are available in Table 1.
临床问题1:成人癌症患者推荐的常规预防性疫苗是什么?
Clinical Question 1: What Are the Recommended Routine Preventative Vaccinations for Adults with Cancer?
自2013年IDSA指南5发布以来进行的研究已涉及新疫苗和接受新型癌症治疗的患者的接种问题。研究人员继续调查癌症治疗期间疫苗的安全性、免疫原性和最佳接种时机。ACIP对成人癌症患者推荐的疫苗总结在表2中。文献回顾和分析主要关注关于这些疫苗推荐用途的新证据。每种疫苗的附加信息,包括副作用,可在CDC的网站上查阅。121在进行诸如HSCT、CAR-T疗法和涉及特定B细胞耗竭治疗的程序后,免疫接种和再免疫需要独特的考虑。这些问题在临床问题2下单独讨论。
Studies conducted since the 2013 IDSA guideline5 have addressed new vaccines and the vaccination of patients receiving new types of cancer therapies. Researchers have continued to investigate the safety, immunogenicity, and optimal timing of vaccinations during cancer treatment. The recommended vaccines from ACIP for adults with cancer are summarized in Table 2. The literature review and analysis primarily focus on new evidence regarding recommended uses of these vaccines. Additional information for each vaccine, including side effects, is available from CDC.121 The approach to immunization and reimmunization after procedures such as HSCT, CAR-T therapy, and treatments involving specific B-cell depletion necessitates unique considerations. These are addressed separately under Clinical Question 2.
包括存在多种有效全身治疗方案在内的治疗方案进步,已促成癌症患者生存率提高。然而,癌症治疗也可能使个体更容易罹患某些感染。某些类型的癌症患者以及接受某些癌症治疗的患者在接种疫苗后可能具有较低的血清转换率。因此,如果可能的话,在治疗开始前最好让癌症患者接种最新的季节性疫苗以及基于年龄和风险的疫苗(最好是在治疗开始前2-4周),从而使他们能够产生足够的免疫应答。这种方法也适用于接种不足或未接种疫苗的成年人,对于该人群而言,补种疫苗的作用变得更加明显。即便由于治疗的紧迫性或持续性而无法在推荐的时间范围内接种疫苗,仍应强烈建议在治疗早期进行疫苗接种,让其获悉可以在癌症治疗期间更灵活地交替接种非活疫苗。
Advances in treatment, including the presence of multiple lines of effective systemic therapy, have resulted in improved survival for patients with cancer. However, cancer therapies may also render individuals at higher risk for some infections. Patients with certain types of cancer, as well as those receiving certain cancer therapies, may have lower seroconversion rates after vaccination. Thus, when feasible, it is of paramount importance that patients with cancer receive up-to-date seasonal vaccines as well as age- and risk-based vaccines (preferably 2-4 weeks) prior to the initiation of treatment, allowing them to mount an adequate response. This approach also applies to under- or unvaccinated adults for whom the role of vaccination catch-up becomes more pronounced. Even if the vaccines cannot be given within the recommended time frame due to the urgency or ongoing nature of the cancer treatment, vaccine administration should still be strongly advised early in the treatment journey acknowledging more flexible intercalation of nonlive vaccines during cancer therapy.
新冠疫苗
COVID-19 Vaccines
文献回顾和分析
Literature Review and Analysis
大量证据涉及新冠疫苗接种后癌症患者的血清学反应。在实体瘤成年患者中,血清转换率往往低于无癌症患者,meta分析报告的相对风险范围为0.90至0.95。22,31,36 血清转换率可能因癌症持续时间和治疗方式而异,近期诊断的患者和接受细胞毒性疗法的患者的转换率较低。在接受免疫检查点抑制剂(ICIs)或靶向疗法的实体瘤患者中,体液反应仅轻微减少。11
A large body of evidence has addressed serologic response to COVID-19 vaccination among patients with cancer. In adults with solid tumors, seroconversion rates tend to be lower than in patients without cancer, with meta-analyses reporting relative risks ranging from 0.90 to 0.95.22,31,36 Seroconversion rates may vary by the duration of cancer and treatment modality, with lower rates observed among recently diagnosed patients and those receiving cytotoxic treatments. Humoral responses are only minimally diminished in patients with solid tumors who are receiving immune checkpoint inhibitors (ICIs) or targeted therapy.11
血清转换率在患有血液癌症的患者中异质性且总体较低。22,30,31,34,37,40 在2022年的一项荟萃分析中,两剂疫苗后的血清转换率分别为:实体瘤患者95%,血液癌症患者64%,慢性淋巴细胞白血病(CLL)患者42%。40 与降低血清转换率相关的治疗措施包括抗CD20剂和其他B细胞定向疗法、Janus激酶(JAK)抑制剂、高剂量皮质类固醇和CAR-T疗法。37,40 特定B细胞靶向治疗后缺乏血清学反应的情况可延续至治疗结束后一年,可能需要重新接种。专家组在这些情况下的处理在临床问题2中进行了讨论。
Seroconversion rates are heterogeneous and overall lower among patients with hematologic cancer than patients with solid tumors.22,30,31,34,37,40 In a 2022 meta-analysis, seroconversion after two vaccine doses was 95% among patients with solid tumors, 64% among patients with hematologic cancers, and 42% in patients with chronic lymphocytic leukemia (CLL).40 Treatments associated with reduced seroconversion rates included anti-CD20 agents and other B-cell–directed therapies, Janus kinase (JAK) inhibitors, high-dose corticosteroids, and CAR-T therapy.37,40 Lack of serological responses after specific B-cell–targeted therapies can extend for up to a year after treatment completion and may warrant revaccination. The panel addresses the approach in these situations in Clinical Question 2.
五项非随机研究支持疫苗接种有助于减少癌症患者患重症新冠感染的风险。62,67,71,75,112 其中一项涉及1,610名患有癌症且新冠病毒检测呈阳性的患者的研究报告称,与未接种疫苗的个体相比,接种疫苗的个体在30天内出现新冠感染住院或死亡的可能性显著降低(比值比为0.44 [95% CI,0.28至0.72])。62 其余研究也报告了接种疫苗与未接种疫苗的成年癌症患者相比,住院率和死亡率的降低67,75,112,或者降低了新冠感染的后遗症率71。
Five nonrandomized studies support the benefit of vaccination in reducing the risk of severe COVID-19 illness in patients with cancer.62,67,71,75,112 One study of 1,610 patients with cancer and a positive COVID-19 test reported that vaccinated individuals were significantly less likely to experience hospitalization for COVID-19 or death within 30 days, compared with unvaccinated individuals (odds ratio, 0.44 [95% CI, 0.28 to 0.72]).62 The remaining studies also report reduced rates of hospitalization and mortality67,75,112 or reduced COVID-19 sequelae71 in vaccinated versus unvaccinated adults with cancer.
新冠疫苗接种后绝大多数不良事件为轻度至中度(1级或2级),最常见的副作用包括接种部位疼痛、疲劳、肌肉疼痛、头痛和发热。41 接种疫苗与短暂的腋窝腺炎有关,乳腺成像学会已就此提供了指导。122
The vast majority of adverse events after COVID-19 vaccination are mild to moderate (grade 1 or 2), with the most common side effects being injection site pain, fatigue, myalgia, headache, and fever.41 Vaccination has been associated with transient axillary adenopathy, and the Society of Breast Imaging has provided guidance on this topic.122
临床解读
Clinical Interpretation
在新冠疫苗出现之前,新冠感染导致了更多的癌症免疫受损患者住院和死亡。71 新冠疫苗保护了癌症患者,降低了患重症新冠感染和住院的风险。62,67,71,75,112 目前最新的建议是,已接种新冠疫苗的个体应接种至少一剂更新的2023-2024年新冠疫苗(任何经授权的抗原)。17 在治疗周期中,没有推荐的最佳接种时间。对于接受已知会削弱疫苗反应的治疗的患者,提供者应强烈推荐在间隔2个月后接种额外的疫苗剂次。对于最近患有新冠感染的个体,建议推迟接种疫苗2-3个月。
COVID-19 resulted in many more hospitalizations and deaths among immunocompromised patients with cancer prior to COVID-19 vaccine availability.71 The COVID-19 vaccines protect patients with cancer, reducing the risk of severe COVID-19 illness and hospitalization.62,67,71,75,112 The most current recommendation for previously COVID-19–vaccinated individuals is to receive at least one dose of the updated 2023-2024 COVID-19 vaccine (any authorized formulation).17 There is no recommended optimal timing during treatment cycles. Providers should strongly recommend additional vaccine doses after a 2-month interval for patients receiving therapies known to weaken vaccine responses. It is recommended to postpone immunization for 2-3 months for individuals who have recently had a COVID-19 infection.
流感疫苗
Influenza Vaccines
文献回顾与分析
Literature Review and Analysis
有9项随机对照试验(RCTs)评估了高剂量和佐剂流感疫苗,以及疫苗接种时间和第二剂疫苗,旨在提高癌症患者对疫苗的应答。6,44-51 高剂量三价流感疫苗与标准剂量三价流感疫苗进行了比较,在一项2016年的试点RCT中,该试验纳入了100名年龄低于65岁、有接受有治疗意愿并在接受化疗的成人。48 高剂量组局部注射部位疼痛更常见。没有发生严重不良事件。高剂量组三价流感抗原的血清转换率更高。两组疫苗的血清保护率没有显著差异。虽然证据有限,但在化疗期间进行流感疫苗接种的RCTs报告称,较早的接种是安全的,并且可能会带来获益。在接受3周细胞毒性化疗的固体肿瘤(主要是乳腺或肺癌)成年人中,第1天或第11天的疫苗接种导致了类似的血清保护率,但第1天的疫苗接种与减少不良反应的风险相关(13%与32%,P = 0.04;大多数不良反应是轻度的)。49 早期(化疗后第5天)和较晚(化疗后第16天)的流感疫苗接种也在一项针对接受乳腺癌或结肠癌化疗的患者的试验中进行了评估;早期接种在仅有乳腺癌的患者中观察到了较高的血清学应答。6
Nine RCTs have evaluated questions such as high-dose and adjuvanted influenza vaccines, as well as vaccine timing, and second vaccine doses with the goal of increasing response to vaccination among patients with cancer.6,44-51 High-dose trivalent influenza vaccine was compared with standard-dose trivalent influenza vaccine in a 2016 pilot RCT that enrolled 100 adults under the age of 65 who were receiving chemotherapy with curative intent.48 Local site pain was more common in the high-dose group. There were no serious adverse events. Seroconversion rates for all three influenza antigens were higher in the high-dose group. Seroprotection did not differ significantly by vaccine group. Although evidence is limited, RCTs of influenza vaccine timing during chemotherapy report that earlier vaccination is safe and may provide benefits. Vaccination on day 1 or day 11 of chemotherapy resulted in similar rates of seroprotection in adults with solid tumors (primarily breast or lung) undergoing 3-week cytotoxic chemotherapy, but day 1 vaccination was associated with a reduced risk of adverse effects (13% v 32%, P = .04; most adverse effects were mild).49 Early (day 5 after chemotherapy) and later (day 16 after chemotherapy) influenza vaccinations were also assessed in a trial of patients undergoing chemotherapy for breast or colorectal cancer; higher serologic response was observed with early vaccination in patients with breast cancer only.6
临床解读
Clinical Interpretation
流感疫苗改善了癌症患者的感染相关结果,建议每年接种,最好在北半球的早秋23 接种。较早的接种可能与季后免疫力逐渐减弱有关。65岁及以上的患者应接种一种优先推荐的高剂量或佐剂流感疫苗方案(高剂量四价疫苗、四价重组流感疫苗和四价佐剂流感疫苗),这些方案已获得对该年龄组患者的许可。检查在癌症治疗期间接种灭活流感疫苗(IIV)的最佳时间的研究有限,并且在与化疗同时进行、在开始时、几天后或在治疗周期之间接种疫苗时的比较结果不一。123-125
Influenza vaccines improve infection-related outcomes in patients with cancer and are recommended annually, ideally to be received by early fall23 in the Northern Hemisphere. Earlier vaccination can be associated with waning immunity later in the season. Patients aged 65 years and older should receive one of the preferentially recommended high-dose or adjuvanted vaccine formulations (high-dose Quadrivalent vaccine, Quadrivalent recombinant flu vaccine, and Quadrivalent adjuvanted flu vaccine) licensed for patients in this age group.123-125
研究限制在癌症治疗期间提供灭活流感疫苗(IIV)的最佳时间的研究有限,并且在比较同时与化疗、在开始时、几天后或在治疗周期之间接种疫苗时的应答时,得出了不同的结论。6,49
Studies examining the optimal timing for administering the inactivated influenza vaccine (IIV) during cancer treatment are limited and have yielded varied conclusions when comparing responses to vaccines administered concurrently with chemotherapy, at initiation, a few days later, or between treatment cycles.6,49
此外,有几项研究探讨了增强IIV免疫原性的不同策略。值得注意的是,高剂量IIV在65岁及以下癌症患者中表现出安全性和优越的免疫原性。48 在50-64岁的一般成年人群中,一项最近的研究显示,与标准剂量相比,高剂量重组疫苗对流感感染的相对疫苗效力较低(15.3% [95% CI,5.9至23.8]),在与流感相关或全因住院和死亡风险上没有显著差异。126 然而,关于高剂量与标准剂量的免疫原性和临床益处的证据仍然有限,难以优先推荐高剂量疫苗给65岁以下的癌症患者。同样,佐剂方案50的证据也没有明显的获益,或者是两剂次疫苗45 的证据也没有明显的获益。总体而言,个体应在可能的情况下接种疫苗,并使用当地能够获取的任何方案。新冠疫苗可以与流感疫苗同时接种,并且在化疗过程中或细胞减少期间同时接种是安全的。最后,鼻喷途径接种的减毒活疫苗不应该被用于癌症患者。
Furthermore, several investigations have explored different strategies to enhance the immunogenicity of IIV. Notably, the high-dose IIV has demonstrated safety and superior immunogenicity in adults aged 65 years and younger with cancer.48 In the general adult population 50-64 years of age, a recent study shows modest relative vaccine effectiveness of the high-dose recombinant vaccine against influenza infection compared to standard-dose (15.3% [95% CI, 5.9 to 23.8]), without significant differences in the risk of influenza-related or all-cause hospitalizations and deaths.126 Nevertheless, evidence remains limited regarding the immunogenicity and clinical benefit of high dose versus standard dose, making it difficult to preferentially recommend the high-dose vaccine for adults with cancer who are below 65 years of age. Similarly, the evidence for adjuvanted formulation50 or a two-dose vaccine series45 does not show clear benefit. In general, individuals should receive the vaccine when possible, with whichever formulation is locally available. The COVID-19 vaccine can be coadministered with the influenza vaccine, and it is safe to vaccinate concurrently with cytotoxic chemotherapy or during the cytopenic period. Finally, the live attenuated influenza vaccine, given as a nasal spray and approved for use in nonpregnant individuals 2-49 years of age, should not be administered to patients with cancer.
乙肝疫苗
Hepatitis B Vaccine
文献回顾与分析
Literature Review and Analysis
系统回顾未发现符合条件的研究。
No eligible studies were identified by the systematic review.
临床解读
Clinical Interpretation
在肿瘤学设置中,许多人在开始某些抗癌治疗之前会接受乙型肝炎筛查,因为在潜在乙型肝炎感染者中存在重新激活的风险。127 尽管在接种乙型肝炎疫苗前并非需要进行特定的检测,但这种筛查实践为评估乙型肝炎免疫力、先前接种情况以及为从未接种过疫苗的个体接种提供了独特的机会。
In oncology settings, many individuals are screened for hepatitis B before initiating certain anticancer therapies due to the risk of reactivation in those with occult hepatitis B infection.127 Although testing is not specifically required prior to hepatitis B vaccination, this screening practice offers a unique opportunity to assess immunity to hepatitis B, previous vaccination status, and to immunize individuals who have never been vaccinated before.
在接受化疗期间接种乙型肝炎疫苗的患者,乙型肝炎表面抗体浓度可能较低。128 乙型肝炎疫苗的加速接种方案以及更新的两剂次和佐剂疫苗制剂在癌症患者或其他免疫受损情况下尚未得到充分评估。CDC 建议免疫受损患者接种更高抗原剂量,如表2所示。129 接种完整第二个系列疫苗已被证明可以提高非应答者的血清保护率。因此,应在最后一剂疫苗接种后1-2个月检查接种后抗表面抗体滴度,如果未达到乙型肝炎表面抗体浓度>10 mIU/mL,应重复接种疫苗系列。
Hepatitis B surface antibody concentrations can be lower in patients who receive hepatitis B vaccine during chemotherapy.128 Accelerated vaccination schedules for hepatitis B and newer two-dose and adjuvanted vaccine formulations have not been thoroughly evaluated in patients with cancer or other immunocompromising conditions. ACIP recommends that immunocompromised patients receive a higher antigen dose as shown in Table 2.129 Administration of a second complete series has been shown to improve the seroprotection rates in nonresponders. Consequently, postvaccination antisurface antibody titers should be checked 1-2 months after the last dose, and the vaccine series should be repeated if hepatitis B surface antibody concentrations >10 mIU/mL are not achieved.
人乳头瘤病毒疫苗
Human Papillomavirus Vaccine
文献回顾与分析
Literature Review and Analysis
系统回顾未发现符合条件的研究。
No eligible studies were identified by the systematic review.
临床解读
Clinical Interpretation
年轻的癌症幸存者患继发性人乳头瘤病毒(HPV)相关恶性肿瘤的发病率比普通人群高。130 造成这种情况的一个重要因素是免疫不足。131 研究表明,HPV疫苗在年轻的癌症幸存者中与普通人群一样免疫原性。何时接种疫苗应基于个体风险。132,133 在接种疫苗之前不需要进行HPV检测或细胞学筛查。
Young cancer survivors have a higher incidence of secondary human papillomavirus (HPV)–associated malignancies when compared to the general population.130 A significant factor contributing to this is underimmunization.131 Studies have revealed that HPV vaccines are as immunogenic in young cancer survivors as they are in the general population. When to administer the vaccine should be based on individual risks.132,133 It is important to note that HPV testing or cytology screening is not required before vaccination.
肺炎球菌疫苗
Pneumococcal Vaccines
文献回顾与分析
Literature Review and Analysis
在一项小型RCT中,患有胃癌和结肠癌的患者在化疗当天或在开始化疗前2周接种疫苗,显示出相当的免疫应答。53
In a small RCT of patients with gastric and colon cancer, vaccination on the day of chemotherapy or 2 weeks before initiation showed comparable immune responses.53
临床解读
Clinical Interpretation
接受治疗的癌症患者患侵袭性肺炎球菌疾病(IPD)的风险明显高于普通人群。患血液恶性肿瘤的患者的风险增加了50倍。134 肺炎球菌疫苗通过降低这一患者群体中肺炎的发生率和住院需求来改善患者预后。66
Patients with cancer undergoing treatment face a significantly higher risk of invasive pneumococcal disease (IPD) compared to the general population. Those diagnosed with hematologic malignancies have a 50-fold elevated risk.134 Pneumococcal vaccination improves patient outcomes by reducing the incidence of pneumonia and the need for hospitalization in this patient population.66
值得注意的是,传统上对多糖疫苗反应较差的患者,如慢性淋巴细胞白血病(CLL)患者,接种多糖蛋白结合疫苗后抗体水平有所提高。然而,接受抗CD20治疗和布氏酪氨酸激酶(BTK)抑制剂治疗的患者的体液反应受到抑制。在开始治疗或γ球蛋白减少症出现前接种疫苗可以获得最强健的免疫应答,强调了早期接种的重要性。52,88,135-138
Notably, patients who have traditionally exhibited poor responses to polysaccharide vaccines, such as those with CLL, demonstrate improved antibody levels with the polysaccharide protein-conjugated vaccines. Still, humoral responses are subdued in patients treated with anti-CD20 therapies and Bruton Tyrosine Kinase (BTK) inhibitors. The most robust immune responses are achieved when the vaccine is administered before starting treatment or before the onset of hypogammaglobulinemia, underscoring the importance of early vaccination.52,88,135-138
通过结合疫苗进行初始启动可增强对随后接种疫苗的抗体反应,为当前推荐的方法提供了信息。在美国,有两种结合疫苗可供选择:15价和20价肺炎球菌结合疫苗(PCV)。对于疫苗未接种的成年癌症患者,可以选择仅接种一剂PCV-20或接种一剂PCV-15后接种一剂PPSV23,两次接种之间至少间隔8周。
Initial priming with a conjugate vaccine enhances the antibody response to subsequently administered vaccines, informing the currently recommended approach. In the United States, two conjugate vaccines are available: pneumococcal conjugate vaccine (PCV)-15 and PCV-20. Vaccine-naive adult patients with cancer can receive either one dose of PCV-20 only or a dose of PCV-15 followed by a dose of PPSV23, with at least 8 weeks between administrations.
重组带状疱疹疫苗
Recombinant Zoster Vaccine
文献回顾和分析
Literature Review and Analysis
美国食品药品监督管理局(FDA)于2017年批准了含有佐剂的重组带状疱疹疫苗(RZV)。这是一种非活性、含有佐剂的重组亚单位(表面糖蛋白E)疫苗。在实体瘤患者56 和血液恶性肿瘤患者55 中,接种两剂疫苗后均能产生免疫应答。在免疫抑制疗法之前或之后接种疫苗时,体液反应往往较高,而在治疗期间接种时则较低。与RZV相关的不良事件很常见。在接种组中,12%-13%的患者报告了3级局部不良事件,如注射部位疼痛,而在安慰剂组中无人报告。55,56 接种组和安慰剂组的患者都报告了3级以上的主动脉疾病,但在接种组中更常见(22%比16%56和16%比6%55)。与新冠疫苗一样,接受BTK抑制剂治疗的患者对RZV的反应较低。103
The adjuvanted, recombinant zoster vaccine (RZV) was approved by the US Food and Drug Administration (FDA) in 2017. It is a nonlive, adjuvanted recombinant subunit (surface glycoprotein E) vaccine. Two doses of the vaccine are immunogenic in patients with solid tumors56 and hematologic malignancies.55 Humoral responses tended to be higher when the vaccine was given before or after immunosuppressive therapy, rather than during therapy. Adverse events were common with RZV. Grade 3 local adverse events, such as injection-site pain, were reported in 12%-13% of patients in the vaccine arms and no one in the placebo arms.55,56 Grade 3, solicited general adverse events were reported by patients in both the vaccine and placebo arms but were more common in the vaccine arms (22% v 16%56 and 16% v 6%55). As is the case for COVID-19 vaccines, response to the RZV was lower in patients treated with BTK inhibitors.103
临床解读
Clinical Interpretation
在癌症诊断后的前两年内,带状疱疹的发生率特别高,而在血液恶性肿瘤患者中,尤其是多发性骨髓瘤患者中,风险最高。此外,与老年成人相比,年轻患者(50岁以下)的癌症相关带状疱疹风险升高更大。139 带状疱疹的并发症,如带状疱疹后遗神经痛,会显著降低生活质量。
The incidence of herpes zoster is particularly high in the first 2 years following a cancer diagnosis, with the greatest risk observed in patients with hematologic malignancies, particularly multiple myeloma. Furthermore, cancer-related herpes zoster risk elevation is greater in younger patients (those below 50 years of age) compared with older adults.139 Complications of herpes zoster, such as postherpetic neuralgia, can significantly diminish quality of life.
美国食品药品监督管理局(FDA)于2017年批准了添加佐剂的重组带状疱疹疫苗(RZV),这标志着科技的重大进步,尤其是考虑到之前的疫苗是一种减毒活疫苗,不推荐给癌症患者使用。RZV应该对所有癌症患者提供。即便在癌症治疗开始后,这种疫苗仍然能保持免疫效果。然而,当疫苗在癌症诊断后立即使用,且在开始免疫抑制治疗之前接种时,可以预期得到最佳的体液和细胞应答。两剂RZV的间隔可以缩短至4周,以实现早期保护。
The approval of the adjuvanted, RZV by the US FDA in 2017 marked a significant scientific advancement, especially since the previous vaccine, a live attenuated formulation, was not recommended for patients with cancer. RZV should be made available to all adults with cancer. The vaccine remains immunogenic even after cancer treatment has begun. However, the most optimal humoral and cellular responses are expected when the vaccine is administered immediately after a cancer diagnosis and before the initiation of immunosuppressive treatments. The interval between the two RZV doses can be reduced to 4 weeks to achieve early protection.
在没有水痘(初次带状疱疹)病史的癌症患者中,尚未研究RZV的效果。曾经经历过带状疱疹的患者应接种疫苗以预防未来的发作。只要急性发作已结束,那么接种疫苗前无需特定的等待时间。在癌症诊断之前可能已接种过带状疱疹减毒活疫苗的患者,也符合接种RZV的资格。最后,目前尚不清楚RZV提供的临床保护持续时间,接种疫苗不应影响某些预防性抗病毒治疗药物(例如,蛋白酶体抑制剂)的使用持续时间。
The RZV has not been studied in patients with cancer who do not have a history of primary varicella (chickenpox). Patients who have experienced herpes zoster should receive the vaccine to prevent future episodes. There is no specific waiting period before immunization, as long as the acute episode has resolved. Patients who may have previously received the live zoster vaccine before cancer diagnosis are eligible to be immunized with the RZV. Finally, the duration of clinical protection from RZV is unclear at this time and vaccination should not influence the duration of antiviral prophylaxis with certain therapies (eg, proteasome inhibitors).
呼吸道合胞病毒疫苗
Respiratory Syncytial Virus Vaccines
文献回顾与分析
Literature Review and Analysis
系统综述未识别到符合条件的研究。
No eligible studies were identified by the systematic review.
临床解读
Clinical Interpretation
60岁及以上的癌症患者有资格接种呼吸道合胞病毒(RSV)疫苗(见表2)。根据美国疾病控制与预防中心(CDC)的指导意见,RSV疫苗可以与其他季节性疫苗接种同时进行。目前没有数据可作为60岁以下癌症患者使用RSV疫苗的参考,因此无法为这个年龄组提出具体建议。
Patients aged 60 years and older with cancer are eligible to receive the respiratory syncytial virus (RSV) vaccine (Table 2). According to the CDC, the RSV vaccine can be coadministered with other seasonal immunizations. There are no data to guide the use of RSV vaccines in patients with cancer younger than 60. No specific recommendation can be made for this age group.
破伤风、白喉和无细胞百日咳疫苗
Tetanus, Diphtheria, and Acellular Pertussis Vaccine
文献回顾与分析
Literature Review and Analysis
系统综述未识别到符合条件的研究。
No eligible studies were identified by the systematic review.
临床解读
Clinical Interpretation
破伤风、白喉和无细胞百日咳(Tdap)的免疫力会随着年龄的增长而减弱,癌症治疗后这种下降可能会加速。140 如果癌症患者成年后没有接种过的话,强烈建议被诊断出癌症的个体接种Tdap疫苗,(见表2)。
Immunity to tetanus, diphtheria, and acellular pertussis (Tdap) tends to decrease with age, and this decline may be accelerated after cancer treatment.140 It is strongly recommended that individuals diagnosed with cancer receive the Tdap vaccine if they have not been vaccinated as adults (Table 2).
成人接受检查点抑制剂(ICI)治疗时的疫苗接种
Vaccination of Adults Receiving ICI Therapy
针对接受检查点抑制剂治疗的患者,疫苗接种是否影响免疫相关不良事件风险的问题已经对流感和新冠疫苗进行了评估。2023年对新冠疫苗接种(主要是mRNA疫苗)的荟萃分析发现,接受ICI治疗的患者与没有癌症的对照组患者的血清转化率相似(相对风险,0.97 [95% CI, 0.92至1.03]39 )。疫苗副作用倾向于轻度或中度,最常见的是局部疼痛和疲劳。与未接种疫苗的个体或历史队列进行回顾性研究没有发现新冠疫苗接种与免疫相关不良事件风险增加有关。141-143 在接受ICI治疗期间接种流感疫苗的患者的两个系统评价也报告了疫苗接种状态的免疫相关不良事件频率差异不显著。33,42
The question of whether vaccination affects the risk of immune-related adverse events in patients receiving checkpoint inhibitor therapy has been evaluated for both influenza and COVID-19 vaccines. A 2023 meta-analysis of COVID-19 vaccination (primarily mRNA vaccines) found that rates of seroconversion were similar in patients receiving ICIs and in a control group of patients without cancer (relative risk, 0.97 [95% CI, 0.92 to 1.03]39). Vaccine side effects tended to be mild or moderate, with the most common being local pain and fatigue. Retrospective studies comparing vaccinated with unvaccinated individuals or historical cohorts have not found an increased risk of immune-related adverse events associated with the COVID-19 vaccines.141-143 Nonsignificant differences in the frequency of immune-related adverse events by vaccination status were also reported in two systematic reviews of patients who received influenza vaccines during ICI therapy.33,42
临床问题2:对于接受造血干细胞移植(HSCT)、CD19 嵌合抗原受体T细胞疗法(CAR-T)治疗或B细胞耗竭疗法的成年患者,推荐哪些额外的疫苗接种和再次接种?
Clinical Question 2: What Additional Vaccinations and Revaccinations Are Recommended for Adults Undergoing HSCT, CD19 CAR-T Treatment, or B-cell–Depleting Therapy?
接受HSCT的患者
HSCT Recipients
众多研究表明,成年HSCT受者会失去儿童时期免疫接种的免疫力,并且在移植后的第一年内特别容易受到可预防疫苗疾病的侵害。再次接种疫苗对于恢复这种免疫力至关重要,最佳的疫苗接种时机基于充分的B细胞和T细胞恢复。各种疾病、移植和受者因素影响免疫恢复和对疫苗的反应,包括受者年龄、供体来源、疫苗类别、移植后间隔、移植抗宿主病(GVHD)预防、免疫抑制持续性、GVHD的严重程度以及疫苗抗原情况。鉴于越来越多使用半相合供体和体内T细胞耗竭以增加供体可用性并减少GVHD的发生率和严重程度,了解上述因素的影响尤为重要,因为这两者都与增加的感染并发症(包括病毒重新激活)相关。
Numerous studies have shown that adult HSCT recipients lose immunity from childhood immunizations and are vulnerable to vaccine-preventable illnesses, particularly in the first year after transplant. Revaccination is essential to restore this immunity, and the optimal vaccine timing is based on adequate B- and T-cell recovery. Various disease, transplant, and recipient factors influence the immunologic recovery and responses to the vaccine, including recipient age, donor source, vaccine type, timing from transplant, graft-versus-host disease (GVHD) prophylaxis, ongoing immunosuppression, GVHD severity, and vaccine antigens. Understanding the influence of these factors has become particularly important given the increasing use of haploidentical donors and in vivo T-cell depletion to increase donor availability and decrease GVHD incidence and severity, as both have been associated with increased infectious complications including viral reactivation.
美国传染病学会(IDSA)、美国疾病控制与预防中心(CDC)、美国移植与细胞治疗学会、欧洲血液和骨髓移植学会(EBMT)以及欧洲白血病感染会议均有关于HSCT受者疫苗接种方法的现有指南。5,144-147 免疫参数如CD19+和CD27+ B细胞(记忆B细胞)计数、免疫球蛋白G水平和CD4计数可用于指导接种时机。然而,目前还没有标准化的方法来应用这些免疫预测指标,也没有关于是否以免疫预测指导的疫苗接种计划比基于移植后时间流逝的标准化计划更有可能诱导保护性免疫反应的相关共识。此外,移植后疫苗接种所获得的保护持久性还需要更好地认知。
The IDSA, CDC, the American Society for Transplantation and Cellular Therapy, European Society for Blood and Marrow Transplantation (EBMT), and European Conference on Infections in Leukemia have existing guidelines on the approach to vaccination in HSCT recipients.5,144-147 Immunologic parameters such as CD19+ and CD27+ B-cell (memory B-cells) count, immunoglobulin G levels, and CD4 count could be used to guide timing. Still, there is no standardized approach for applying these immune predictors, nor is there consensus on whether immune predictor–guided vaccination schedules are more likely to induce a protective immune response than a standardized schedule based on the time elapsed after transplantation. Furthermore, the durability of protection attained by post-transplant vaccination needs to be better understood.
新冠疫苗
COVID-19 Vaccines
文献回顾与分析
Literature Review and Analysis
对HSCT(造血干细胞移植)受者进行的初级mRNA疫苗评估显示,这些疫苗在这些个体中能够引发免疫反应;然而,与其他群体相比,总体体液免疫反应较低,研究显示无论是异基因(allo)还是自体(auto)HSCT的体液反应率在79.6%-86.1%之间。与健康志愿者和实体瘤患者相比,接受HSCT的患者的新冠感染抗体和细胞反应较低。在HSCT后的第一年内,体液反应受到抑制,这一效应随着年龄的增长、并发淋巴细胞减少症、GVHD、潜在的非霍奇金淋巴瘤以及持续使用免疫抑制剂或皮质类固醇而更加明显。57,95,99 证据还表明,通过增加初级和加强剂次可以改善血清转换,以应对HSCT受者初期反应不佳和抗体衰减加速的问题。148
Assessments of primary mRNA vaccines among HSCT recipients demonstrate vaccines elicit an immune response in these individuals; however, the overall humoral response is lower compared to other groups, with studies showing a range of 79.6%-86.1% for both allogeneic (allo) and autologous (auto) HSCT. COVID-19 antibody and cellular responses are lower in patients undergoing HSCT than healthy volunteers and patients with solid tumors. Humoral responses are subdued in the first year after HSCT, and this effect is more pronounced with advanced age, concurrent lymphopenia, GVHD, underlying non-Hodgkin lymphoma, and ongoing immunosuppressive or corticosteroid use.57,95,99 Evidence also indicates that seroconversion is improved with additional primary and booster doses to address poor initial response and accelerated antibody decay in HSCT recipients.148
一部分体液无反应者在接种疫苗后能够产生针对病毒的特异性细胞免疫反应。一项多中心前瞻性研究支持在4个月前进行异基因HSCT的早期新冠疫苗接种,与在4至12个月间开始接种疫苗的人相比,无论是体液还是细胞反应均相当,包括那些有GVHD和正在接受免疫抑制治疗的患者。77 关于新冠疫苗接种后移植抗宿主病(GVHD)爆发的报告对因果关系的结论不确定,且没有出现其他重大安全问题。57,77,80
A substantial subset of humoral nonresponders can mount virus-specific cellular immune responses after vaccination. A single multicenter prospective study of allo HSCT supports early COVID-19 vaccination before 4 months with comparable humoral and cellular responses to those starting vaccination between 4 and 12 months, including among those with GVHD and ongoing immunosuppression.77 Reports of GVHD flares after COVID-19 vaccination are inconclusive for a causal association, and no other significant safety concerns have emerged.57,77,80
临床解读
Clinical Interpretation
随着疫苗、抗病毒药物和免疫治疗药物用于治疗和预防新冠感染的可用性,HSCT(造血干细胞移植)患者的新冠感染相关死亡率有所改善。与免疫功能正常的个体相比,疫苗在HSCT受者中的有效性较低,并且特别是在HSCT后的第一年内效力下降更快。研究一致显示,mRNA疫苗在HSCT受者中具有免疫原性。尽管如此,两剂系列的反应与普通人群和接受实体瘤治疗的患者相比较低。其他负面影响疫苗免疫原性的因素包括年龄较大、淋巴细胞减少症、皮质类固醇的使用和慢性GVHD。淋巴系统恶性肿瘤,尤其是HSCT之前接受过抗CD20治疗的,是另一个导致体液反应较弱的重要因素。总体而言,自体移植后的反应,尤其是在多发性骨髓瘤患者中,高于异基因HSCT后观察到的反应。
COVID-19–related mortality in HSCT has improved with the availability of vaccines, antivirals, and immunotherapeutics to treat and prevent COVID-19. Vaccine effectiveness is lower in HSCT recipients when compared to immunocompetent individuals and wanes more rapidly, particularly in the first year after HSCT. Studies have consistently shown that mRNA vaccines are immunogenic in HSCT recipients. Still, a two-dose series elicits lower responses when compared to the general population and patients undergoing treatment for solid tumors. Other factors adversely influencing vaccine immunogenicity are older age, lymphopenia, corticosteroid use, and chronic GVHD. Lymphoid malignancies, especially for which anti-CD20 therapies precede HSCT, are another critical determinant of a less robust humoral response. Overall, responses after autologous transplant, especially among those with multiple myeloma, are higher than those observed after allogeneic HSCT.
即使在缺乏血清转换的HSCT患者中,也能激发T细胞反应,这可能在保护免受严重疾病的作用中扮演重要角色,特别是当体液免疫受损时。在基础疫苗程序中加入第三剂疫苗可以增强细胞介导的反应和抗体滴度,因此目前推荐在HSCT后再次进行疫苗接种。移植后尽早接种疫苗是可行的,因为从移植到接种的时间间隔较短与新冠感染较差结局相关。至少有一项关于异基因HSCT受者的研究显示了疫苗接种能够提供良好的免疫反应,包括在急性GVHD患者中,77 这支持了美国和欧洲领先的移植专业社团推荐在HSCT后3个月开始接种疫苗的当前建议。疫苗接种应使用公共卫生机构推荐的最新抗原疫苗完成。
cell responses are elicited in a subset of HSCT patients despite a lack of seroconversion and may play an essential role in protection against severe disease, especially when humoral defenses are impaired. A third vaccine dose in the primary series can boost cell-mediated responses and antibody titers and is therefore the currently recommended approach for revaccination after HSCT. Vaccination earlier after transplant is desirable, as a shorter time interval from transplant correlates with worse COVID-19 outcomes. At least one study of allogeneic HSCT recipients showed good immune responses, including among patients with acute GVHD,77 lending support to the current recommendations by leading US and European transplant professional societies to start vaccination at 3 months after HSCT. Vaccination should be completed with the most updated formulation recommended by public health agencies.
捐献者接种疫苗既不切实际也无优势,且伦理考量增加了进一步的复杂性。关于mRNA疫苗作为GVHD触发因素的数据导致了不同的结论。HSCT中加速的免疫衰退是一个正在进行的研究领域,旨在优化加强剂次的时机和剂次。
Donor vaccination is neither practical nor advantageous over current approaches, and ethical considerations add further complexity. The data on mRNA vaccines as a trigger for GVHD have led to different conclusions. Accelerated immune decay in HSCT is an area of ongoing investigation to optimize the timing and number of booster doses.
流感疫苗
Influenza Vaccines
文献回顾与分析
Literature Review and Analysis
研究表明,尽管HSCT(造血干细胞移植)受者的血清学反应较低,但季节性流感疫苗(IIVs)仍能提供显著的临床保护。87,100 通常在HSCT后6个月接种流感疫苗,但由于感染的季节性特征,也可以在HSCT后3-6个月之间的社区传播高峰期间提前接种。随机对照试验(RCTs)比较了成人异基因HSCT受者使用佐剂和非佐剂流感疫苗的血清转化率,显示出类似的免疫原性。50 两项在成人异基因HSCT受者中进行的RCT,比较了单剂和两剂高剂量与标准剂量疫苗的抗体滴度,结果显示高剂量流感疫苗对H3N2和H1N1抗原以及H3N2和B Victoria抗原的抗体滴度高于标准剂量。47,149 使用两剂疫苗接种方案接种的自体HSCT受者,初始剂量为高剂量或标准剂量,随后两组均接种了第二剂标准剂量(SD),在移植后平均2.3个月获得了高血清保护率,针对所有流感抗原的保护率在75.8%到97.1%之间。51 相比之下,在异基因HSCT受者中,尤其是在HSCT第一年内接种的个体,接种两剂标准剂量IIV后,并没有显著增加对所有流感抗原的血清保护或血清转化。44 两剂高剂量IIV在3至17岁的儿童HSCT受者中安全且显示出比标准剂量更优越的免疫原性,尤其是针对甲流抗原。150
Studies have shown that IIVs offer significant clinical protection, despite low serological responses among HSCT recipients.87,100 Influenza vaccine is generally administered 6 months post-HSCT, but due to the seasonal nature of the infection, can be given earlier, between 3-6 months post–auto- or allo-HSCT during periods when high community transmission is expected. RCTs have compared the seroconversion rates with adjuvanted and nonadjuvanted influenza vaccines in adult allogenic HSCT recipients demonstrating similar immunogenicity.50 Two RCTs in adult allogenic HSCT recipients comparing single and two high-dose versus standard-dose formulations show higher antibody titers after the high-dose influenza vaccine compared to the standard dose to H3N2 and H1N1 antigens and H3N2 and B Victoria antigens, respectively.47,149 Auto-HSCT patients vaccinated at a median of 2.3 months after transplant achieved a high seroprotection rate for all influenza antigens using a two-dose approach with an initial high-dose or standard dose followed by a second SD in both arms, ranging from 75.8% to 97.1%.51 In contrast, no significant increase in seroprotection or seroconversion for all influenza antigens occurred in allo-HSCT recipients after two doses of standard-dose IIV, especially in those vaccinated within the first year of HSCT.44 Two high-dose IIV doses were safe and showed superior immunogenicity for influenza A antigens compared to standard dose in pediatric HSCT recipients ages 3-17 years.150
临床解读
Clinical Interpretation
高剂量流感疫苗安全且免疫原性优于标准剂量疫苗。因此,对成人HSCT受者来说,无论年龄大小,都更倾向于使用高剂量IIV。支持标准或高剂量两剂方案优越性的数据较不充分。
The high-dose influenza vaccine is safe and has improved immunogenicity compared to the standard-dose vaccine. Thus, high-dose IIV is preferred in adult HSCT recipients regardless of age. The data supporting the superiority of a two-dose regimen of standard or high doses are less robust.
肺炎球菌疫苗
Pneumococcal Vaccines
文献回顾与分析
Literature Review and Analysis
HSCT(造血干细胞移植)受者在移植后早期对有荚膜细菌的感染风险极高,尤其在HSCT后的第一年内,侵袭性肺炎球菌病(IPD)的发病率居高不下。109 引入PCV(肺炎球菌结合疫苗)显著减少了HSCT受者中的IPD。106 对移植后患者的研究显示,64%-98%的受者对包含的血清型产生了抗体反应,这些反应因疫苗类型、移植后时间以及与GVHD(移植物抗宿主病)相关的免疫抑制情况而异。为减轻早期IPD的风险,一个从移植后3个月开始的13价肺炎球菌结合疫苗(PCV-13)接种计划显示出与晚期接种方案(从9个月开始)相当的功能性抗体水平。70 然而,较早的接种与保护作用的减弱和GVHD患者中较低的反应相关。70 总体而言,比较早期与晚期肺炎球菌免疫接种的研究显示了类似的初始抗体反应,但早期组免疫力在24个月时显著下降。82
HSCT recipients are at exceptionally high risk for infections with encapsulated bacteria early after transplant and have among the highest incidence of IPD, especially in the first year after HSCT.109 Introduction of the PCV has significantly reduced IPD in HSCT recipients.106 Post-transplant studies show that 64%-98% of recipients develop antibody responses against covered serotypes, varying by vaccine type, time from transplant, and GVHD-associated immunosuppression. To mitigate the risk of early IPD, a pneumococcal 13-valent conjugate vaccine (PCV-13) schedule beginning at 3 months post-transplant revealed a comparable level of functional antibodies to late immunization beginning at 9 months.70 However, earlier vaccination was associated with waning protection and lower responses in patients with GVHD.70 Overall, studies that have compared early versus late pneumococcal immunization show similar initial antibody responses but a significant decline by 24 months in the early group.82
虽然没有关于在HSCT受者中使用20价肺炎球菌结合疫苗(PCV-20)的研究,且实际经验有限,但目前美国的建议是在移植后4-6个月给所有HSCT受者接种第一剂PCV-20,因为这增加了对额外肺炎球菌血清型的覆盖。74,151 接下来的两剂以1个月间隔接种,随后第四剂在第三剂6个月后接种。已经开始用PCV-15重疫苗接种的患者可以用PCV-20完成后续剂次。然而,对于PCV-15系列,可以在第四剂PCV-15后2个月提供PPSV-23(肺炎球菌多糖疫苗)。无论有无GVHD的HSCT幸存者从结合疫苗中获得的长期免疫仍未确定。
Although no studies are available with the pneumococcal 20-valent conjugate vaccine (PCV-20) in HSCT recipients and the practical experience with this approach is limited, the current US recommendation is to revaccinate all HSCT recipients with the first dose of PCV-20 at 4-6 months after transplant since this adds coverage for additional pneumococcal serotypes.74,151 The subsequent two doses are given at 1-month intervals, followed by the fourth dose administered 6 months later. Patients who have started revaccination with PCV-15 can complete subsequent doses with PCV-20. However, for the PCV-15 series, PPSV-23, a pneumococcal polysaccharide vaccine, can be administered 2 months after the fourth PCV-15 dose. The long-term immunity from conjugated vaccines in HSCT survivors with and without GVHD remains undetermined.
临床解读
Clinical Interpretation
从移植后3个月开始,对HSCT(造血干细胞移植)受者进行较早的再次接种是首选方案,并在1年后接种第四剂结合加强剂次。尽管相关证据仍然有限,但结合疫苗的免疫原性改善和血清型覆盖范围的扩大使得PCV-20疫苗更受青睐。监测社区中循环的肺炎球菌血清型并审查及时的国家指南将有助于确定在20价肺炎球菌结合疫苗之后是否需要使用PPSV-23疫苗来覆盖额外的血清型。将来需要明确额外剂次的益处。69
Earlier revaccination of HSCT recipients starting after 3 months is the preferred approach in combination with a fourth conjugate vaccine dose administered at 1 year. Improved immunogenicity of the conjugated vaccine and the expanding serotype coverage have led to preference for the PCV-20 vaccine, although evidence remains limited. Surveillance of circulating pneumococcal serotypes in the community and review of timely national guidelines will help determine whether a PPSV-23 boost is necessary after the pneumococcal 20-valent conjugate vaccine to cover the additional serotypes. The benefit of administering additional doses needs to be defined in the future.69
重组带状疱疹疫苗
RZV
文献回顾与分析
Literature Review and Analysis
在HSCT(造血干细胞移植)关键的带状疱疹效力试验中,疫苗受种者展示了强大的抗糖蛋白抗体和细胞反应。在接受自体HSCT的患者中,那些在移植后50至70天接种疫苗的,在中位数21个月时对带状疱疹的整体临床效果为68.2%(95% CI,56至78)。54,152 随访评估显示,在接种疫苗后2年的持续临床疫苗效力,包括对多发性骨髓瘤患者的72%和对非霍奇金淋巴瘤患者的61%,在18-49岁和50岁及以上的患者之间没有显著差异。153 在接种疫苗后2年,体液反应显著下降,且在非霍奇金淋巴瘤患者中总体较低;然而,强大的细胞介导免疫反应得以保留,并在两个年龄组和疾病类型之间具有可比性,153 这表明疫苗产生的多功能T细胞免疫提供了对带状疱疹的主要免疫保护。在异基因HSCT中对RZV的评估限于小型研究且免疫测量不完整,但疫苗安全且无加重GVHD(移植物抗宿主病)的风险。59 最后,虽然RZV未获批准用于预防初发水痘,但证据报告表明这是一种安全有效的策略。
The pivotal Zoster Efficacy in HSCT trial demonstrated robust antiglycoprotein antibody and cellular responses in vaccine recipients. In auto HSCT recipients who received the vaccine between 50 and 70 days after transplant, the overall clinical efficacy was 68.2% (95% CI, 56 to 78) for herpes zoster prevention at a median of 21 months.54,152 Follow-up evaluations showed sustained clinical vaccine effectiveness at 2 years after vaccination, including 72% for patients with multiple myeloma and 61% for those with non-Hodgkin's lymphoma, without significant difference in patients 18-49 years old and those 50 and older.153 Humoral responses declined significantly at 2 years after vaccination and were overall lower in patients with non-Hodgkin's lymphoma; however, robust cell-mediated immune responses were retained and comparable across both age groups and disease type,153 suggesting vaccine-generated polyfunctional T-cell immunity renders primary immune protection against herpes zoster. The RZV assessments in allogenic HSCT are limited to small studies with incomplete immunologic measurements, but the vaccine is safe without the risk of GVHD exacerbation.59 Finally, while the RZV is not approved for the prevention of primary varicella, the evidence reports this as a safe and effective strategy.
临床解读
Clinical Interpretation
尽管没有可用数据指导异基因HSCT中RZV的使用,但疫苗可以在抗病毒预防结束后接种,通常在异基因移植后12-18个月以及自体移植后3-12个月接种。如果有迹象表明有需要,例如慢性GVHD或与移植或其他合并症相关的持续免疫抑制,应继续延长抗病毒预防。
Although no data are available to guide the use of RZV in allo-HSCT, the vaccine may be administered after the end of antiviral prophylaxis, usually 12-18 months after an allogeneic and 3-12 months after an autologous HSCT. Antiviral prophylaxis should be continued longer if there is an indication, such as chronic GVHD or ongoing immunosuppression related to transplant or other comorbidities.
乙型肝炎疫苗
Hepatitis B Vaccine
文献回顾与分析
Literature Review and Analysis
推荐在HSCT(造血干细胞移植)后6到12个月间接种三或四剂重组乙型肝炎疫苗(见表2)。在自体和异体HSCT后第一年的再疫苗接种血清转换率在64%到100%之间,年龄较大的个体和有GVHD(移植物抗宿主病)的患者中转换率较低。154 在接受过移植的、隐匿性乙型肝炎感染的受者(核心抗体阳性;表面抗原阴性)中,疫苗接种后的仍存在乙肝病毒活跃风险,无论抗乙型肝炎表面抗体水平如何,累积3年风险为29%。94,113,155
Vaccination with three or four doses of the recombinant hepatitis B vaccine (Table 2) is recommended between 6 and 12 months after HSCT. Seroconversion rates with revaccination in the first year after auto- and allo-HSCT have ranged from 64% to 100% and are lower among older individuals and those with GVHD.154 Reactivation after post-transplant immunization of recipients with resolved hepatitis B infection (core antibody positive; surface antigen negative) can happen irrespective of anti–hepatitis B surface antibody levels and with a cumulative 3-year risk of 29%.94,113,155
临床解读
Clinical Interpretation
乙型肝炎疫苗对HSCT(造血干细胞移植)患者来说是安全且能有效激发免疫反应的。对于那些在移植后12个月内接种疫苗的患者,应在接种后6个月定期检查乙型肝炎表面抗体滴度,并且如果抗体水平低于保护阈值(乙型肝炎表面抗体小于10 mIU/mL),应重复进行三剂系列疫苗接种。
Hepatitis B vaccines are safe and immunogenic in HSCT patients. Hepatitis B surface antibody titers should be routinely checked 6 months postimmunization, especially for those vaccinated within 12 months of transplant, and a three-dose series repeated for levels below the protective threshold (hepatitis B surface antibody <10 mIU/mL).
此外,对于那些隐匿性乙型肝炎感染的患者(核心抗体阳性,表面抗原阴性),在停止抗病毒治疗后可能会出现晚期并发症,即病毒重新激活,其在2年内的累积风险高达40%。156-158 虽然高水平的表面抗体与较低的重新激活风险相关,159 但目前尚不清楚是否通过增加HSCT捐献者和受者的抗体水平的免疫接种能够为有隐匿性乙型肝炎感染的HSCT受者提供持久的临床保护。157
Viral reactivation, in patients with occult hepatitis B infection (core antibody–positive, surface antigen–negative), can be a late complication after antiviral cessation, with a 2-year cumulative risk of up to 40%.156-158 Although high surface antibody levels correlate with a lower risk of reactivation,159 it remains unclear whether immunization of HSCT donors and recipients to increase antibody levels confers durable clinical protection against reactivation in HSCT recipients with occult hepatitis B infection.157
白喉、破伤风、百日咳、脊髓灰质炎和B型流感嗜血杆菌
Diphtheria, Tetanus, Pertussis, Polio, and Haemophilus B Influenza
文献回顾与分析
Literature Review and Analysis
通常推荐在移植后6至12个月开始重新接种这些儿童时期的疫苗,无论是否持续进行免疫抑制治疗。一份报告中提到,84名接受异基因HSCT的受者在中位数369天(86%在100至500天之间)接种了白喉、破伤风、百日咳、脊髓灰质炎和B型流感嗜血杆菌(DTaP-IPV-Hib)疫苗,其中13%有活跃的GVHD,接种后血清学反应如下:B型流感嗜血杆菌97.4%;白喉88%;破伤风95.2%;百日咳68.3%。107 大多数对百日咳疫苗产生免疫应答者对所有其他疫苗抗原都有能产生保护性抗体(96.4%-100%)。在无反应者中,88.9%之前有GVHD,而在有反应者中仅有54.7%。107 最近一项研究评估了成人异基因HSCT患者接种包括白喉、破伤风、无细胞百日咳、乙型肝炎、灭活脊髓灰质炎病毒和B型流感嗜血杆菌(DTaP-HB-IPV-Hib)的儿童联合疫苗,其中三分之一的参与者有慢性GVHD,并在移植后中位数12个月重新接种疫苗。结果显示,除了乙型肝炎外,移植后2年所有抗原的血清学反应均超过90%。68 现有数据支持含有高抗原剂量的疫苗能提供更优越的免疫反应并且安全。在美国,成人HSCT受者可以接种三剂DTaP或Tdap,然后接种两剂白喉和破伤风疫苗(DT或Td)。在欧洲移植中心,成人移植受者常规重新接种DTaP已成为标准做法。107
Generally, revaccination with these childhood immunizations is recommended starting 6-12 months after transplant regardless of ongoing immunosuppression. A report of 84 allo-HSCT recipients immunized with Diphtheria, Tetanus, Pertussis, Polio, Haemophilus B Influenza (DTaP-IPV-Hib) at a median of 369 days (86% between 100 and 500 days), including 13% with active GVHD, reported serological responses as follows: Haemophilus B Influenza 97.4%; diphtheria 88%; tetanus 95.2%; and pertussis 68.3%.107 Most pertussis vaccine responders had protective antibodies to all other vaccine antigens (96.4%-100%). Prior GVHD was present in 88.9% of the nonresponders, and only 54.7% of the responders.107 A recent study evaluated combined pediatric formulations of diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus, and Hemophilus influenzae (DTaP-HB-IPV-Hib) in adults who underwent allo-HSCT, including a third of the participants with chronic GVHD and were reimmunized at a median of 12 months after transplant. Results showed >90% serological responses sustained through 2 years after transplant for all antigens except hepatitis B.68 Existing data support that high antigen dose-containing vaccines give superior immune responses and are safe. In the United States, adult HSCT recipients can receive either three doses of DTaP or Tdap followed by two doses of Diphtheria and Tetanus vaccine (DT or Td). Routine revaccination of adult transplant recipients with DTaP is standard practice at European transplant centers.107
临床解读
Clinical Interpretation.
移植受者可能需要接种加强剂次疫苗,因为患者随时间流逝会失去保护性抗体水平,尤其是对白喉。160
Booster doses are likely to be needed in transplant recipients since patients lose protective antibody levels over time, especially against diphtheria.160
脑膜炎球菌结合疫苗
Meningococcal Conjugate Vaccine
文献回顾与分析
Literature Review and Analysis
一项针对67名接受自体和异体HSCT(造血干细胞移植)的受者接种脑膜炎球菌结合疫苗的研究显示,其中59%的患者有GVHD(移植物抗宿主病),在没有预先免疫的情况下,针对特定血清群的反应率为:A群77%,C群65.5%,W-135群52%,Y群65%。60,64
A study of 67 auto- and allo-HSCT recipients vaccinated with the meningococcal conjugate vaccinate, of whom 59% had GVHD, demonstrated serogroup-specific responses in those without pre-existing immunity of 77% (serogroup A); 65.5% (serogroup C); 52% (serogroup W-135); and 65% (serogroup Y).60,64
临床解读
Clinical Interpretation
推荐在移植后6至12个月,为有风险因素的移植受者接种两剂四价脑膜炎球菌疫苗,两剂之间间隔2个月。也应向有高风险状况的HSCT受者或有资格接种疫苗的年轻成人(16-23岁)提供B群脑膜炎球菌疫苗。移植受者可能需要加强剂次,因为患者随时间流逝会失去保护性抗体水平。
Two doses of quadrivalent meningococcal vaccine 2 months apart are recommended 6-12 months after transplant for recipients with risk factors. Meningococcal B vaccines should also be offered to HSCT recipients with high-risk conditions or young adults (16-23 years old) who are eligible to receive the vaccine. Booster doses are likely to be needed in transplant recipients since patients lose protective antibody levels over time.
人乳头瘤病毒疫苗
HPV Vaccine
文献回顾与分析
Literature Review and Analysis
HPV相关癌症常见于HSCT幸存者中。161,162 移植前的HPV感染和GVHD的发展与移植后多发性HPV相关上皮细胞增生的高负担相关,5年和10年的累积风险分别为28.1%和36.7%。161 在一项针对44名接受异体HSCT的女性的研究中,接种四价HPV疫苗后的抗体反应与健康对照组相当,其中半数患者正在接受系统性免疫抑制治疗。疫苗耐受性良好,副作用轻微,且没有加剧GVHD。111
HPV-associated cancers often occur among HSCT survivors.161,162 The combination of HPV infection prior to transplant and development of GVHD are associated with a high burden of post-transplant multifocal HPV-associated epithelial hyperplasia, with a 5- and 10-year cumulative risk of 28.1% and 36.7%, respectively.161 The quadrivalent HPV vaccine, when given at a median of 2.5 years from transplant, induced antibody responses comparable to healthy controls in a study of 44 women who had undergone allo-HSCT, wherein half of the patients were receiving systemic immunosuppression. The vaccine was well tolerated with mild side effects and without GVHD exacerbation.111
临床解读
Clinical Interpretation
HPV疫苗在HSCT受者中的免疫原性尚未得到充分研究。重新接种疫苗对年轻的移植受者(19-45岁)尤其重要,这些人不仅HPV暴露的风险增加,而且与普通人群相比,发展HPV相关癌症的风险也更大。普遍推荐19至45岁成人接种HPV 9价疫苗。163 对于已感染HPV的年长者,移植后接种疫苗是否能降低HPV相关癌症的风险目前尚不清楚,这是一个需要研究的领域。HPV疫苗接种的最佳时机在HSCT后尚未确定,可以在9至12个月开始接种。
Immunogenicity of HPV vaccines has not been well studied in HSCT recipients. It is especially important to revaccinate young (age 19-45 years) transplant recipients who are not only at heightened risk of HPV exposure but are also at a greater risk of developing HPV-related cancers compared to the general population. The HPV 9-valent vaccine is broadly recommended for adults up to age 45 years.163 Whether older already-HPV–infected individuals will have a reduced risk of HPV-associated cancers from post-transplant vaccination is currently unknown and an area for study. The optimal timing for HPV vaccination after HSCT is not established and can be initiated around 9-12 months.
呼吸道合胞病毒疫苗
RSV Vaccines
文献回顾与分析
Literature Review and Analysis
系统综述未识别到符合条件的研究。
No eligible studies were identified by the systematic review.
临床解读
Clinical Interpretation
最近在多个国家为60岁及以上的下呼吸道RSV感染高风险成人批准了两种RSV疫苗。关键试验并未包括免疫功能受损的患者,且目前没有关于HSCT患者的免疫原性数据,也缺乏指导细胞治疗后RSV疫苗的接种时间或剂量的数据。
Two RSV vaccines were recently licensed in multiple countries for adults aged 60 and older who are at high risk for RSV lower respiratory tract infection. Pivotal trials did not include immunocompromised patients and there are no data available on immunogenicity in HSCT, or to guide the timing or doses of RSV vaccines after cellular therapies.
减毒活疫苗:麻疹风疹腮腺炎联合疫苗和水痘疫苗
Live Vaccines: MMR and Varicella
麻疹风疹腮腺炎联合疫苗
MMR
MMR抗体水平在移植后显著下降,特别是在那些通过疫苗获得免疫的人中。164 MMR是常规推荐给血清阴性移植受者的活疫苗。疫苗接种应在HSCT后至少2年,前提是没有出现GVHD。应对有缓解GVHD的患者进行个体评估以确定接种时间。此外,患者在接种疫苗前8-11个月不应接受系统性免疫抑制剂或静脉注射免疫球蛋白(IVIG)。
MMR antibody levels wane significantly after transplant, especially among those with vaccine-induced immunity.164 MMR is a routinely recommended live vaccine for seronegative transplant recipients. The vaccine should be given no sooner than 2 years after HSCT, provided there is no occurrence of GVHD. Individual assessment should be made to determine the timing for patients with resolving GVHD. Additionally, the patient should not have received systemic immunosuppressives or intravenous immunoglobulin (IVIG) for 8-11 months prior to vaccine administration.
水痘疫苗
Varicella
可以为没有原发性水痘史且血清阴性的患者接种两剂水痘疫苗,两剂间隔1个月,接种时间不早于HSCT后2年,且在没有GVHD、至少一年没有使用系统性免疫抑制剂和8-11个月没有接收IVIG的情况下进行。尽管研究表明疫苗接种可以在血清阴性的实体器官移植患者中诱导体液和细胞免疫反应,但目前没有数据表明重组带状疱疹疫苗(RZV)可以在血清阴性HSCT患者中预防水痘。165
A two-dose series of varicella vaccines administered 1 month apart may be given to varicella-seronegative patients without a history of primary varicella, no sooner than 2 years after HSCT and in the absence of GVHD, no systemic immunosuppressive use for least a year, and no receipt of IVIG for 8-11 months. There are currently no data regarding the efficacy of RZV for protection against varicella in seronegative HSCT patients although it was shown that vaccination could induce both humoral and cellular immune responses after solid organ transplantation in seronegative patients. 165
嵌合抗原受体T细胞疗法(CAR-T)受者
Chimeric Antigen Receptor T-Cell Therapy Recipients
嵌合抗原受体(CAR)T细胞疗法是一种涉及采用细胞转移的免疫疗法。目前批准用于治疗B细胞白血病、非霍奇金淋巴瘤(NHL)和多发性骨髓瘤,这种治疗与淋巴细胞消耗和持久的B细胞再生不良相关。
Chimeric antigen receptor (CAR) T-cell therapy is a form of immunotherapy that involves adoptive cell transfer. Currently approved for treating B-cell leukemias, non-Hodgkin lymphoma (NHL), and multiple myeloma, this treatment is associated with lymphodepletion and long-lasting B-cell aplasia.
文献回顾与分析
Literature Review and Analysis
接种新冠疫苗后,发展体液免疫反应的频率在28.2%到35.9%之间,接种时间在CAR-T治疗前和治疗后6个月的反应率相似。28,166,167 多达72.2%的受者发展了可提供保护性免疫的细胞反应。62 正如其他疫苗观察到的,与NHL患者相比,骨髓瘤患者在CAR-T后的反应更好。额外的疫苗剂量在长期B细胞再生不良的患者中是安全的,但对抗体水平的提升作用有限。类似地,接受流感疫苗的CAR-T缓解期受者在治疗后13-57个月有至少四倍抗体滴度增加。116
The frequency of developing humoral responses to COVID-19 vaccines ranges from 28.2% to 35.9%, with similar response rates when vaccinated before and 6 months after CAR-T treatment.28,166,167 Up to 72.2% of recipients develop cellular responses that can confer protective immunity.62 As observed with other vaccines, responses are better in post–CAR-T patients with myeloma compared to those with NHL. Additional vaccine doses are safe but have a modest effect on antibody levels in those with long-lasting B-cell aplasia. Similarly, 31% of CAR-T recipients in remission vaccinated against influenza 13-57 months after treatment had at least fourfold increases in antibody titers for ≥1 vaccine antigens.116
Walti等168 对54名接受CAR-T治疗的患者进行了评估,其中大多数患者患有非霍奇金淋巴瘤。这些患者先前平均经历了五种治疗方案,58%的患者之前接受过HSCT,54%的患者最近接受过IVIG治疗。在CAR-T治疗后平均20个月检测的针对疫苗抗原的抗体水平与美国成人人群相当,即使是那些最近没有接受IVIG的患者也是如此。疫苗接种后肺炎球菌、Hib、百日咳(0%-15%)和乙型肝炎(39%)的抗体水平最低。CAR-T后6个月内对PCV的反应被发现是次优的。169
Walti et al168 evaluated 54 CAR-T recipients, most of whom had non-Hodgkin's lymphoma. These patients had undergone a median of five prior lines of therapy, 58% had previously undergone HSCT, and 54% had received recent IVIG. Measured antibody levels against vaccine antigens at a median of 20 months post–CAR-T treatment were comparable to the US adult population, even for patients without recent IVIG. Antibody levels were lowest for pneumococcus, Hib, pertussis (0%-15%), and hepatitis B (39%). Responses to the PCV were found to be suboptimal within 6 months of CAR-T.169
临床解释
Clinical Interpretation
针对接受CAR-T治疗的血液恶性肿瘤患者明确疫苗接种指标的研究较为有限。因此,非活疫苗的接种最好在CAR-T治疗前或至少在治疗后6-12个月进行。流感和新冠疫苗理想情况下应在淋巴细胞减少前2周接种,或遵循与HSCT患者相同的时机(CAR-T治疗后≥3个月)。目前没有数据指导活疫苗的安全性和接种时机。CAR-T受者的疫苗重新接种需求和其他疫苗的接种时机仍不明确。当前的文献有限,主要来源于针对CD19靶点的治疗,并且正逐渐涵盖接受B细胞成熟抗原CAR-T治疗的个体。疫苗反应的异质性相当大,主要受治疗靶向抗原结构和B细胞表达此抗原的分化阶段的影响。除了宿主相关因素(如高龄、之前的治疗方案),长期的治疗相关B细胞无力症、细胞减少症和低丙种球蛋白血症也会导致疫苗体液免疫反应减弱。
Studies are limited from which to define vaccination metrics for patients receiving CAR-T therapy for hematologic malignancies. Thus, administration of nonlive vaccines preferably should occur before CAR-T treatment or at least 6-12 months thereafter. Influenza and COVID-19 vaccines ideally should be given 2 weeks before lymphodepletion or follow the same timing as recommended for HSCT patients (≥3 months post–CAR-T treatment). There are no data to guide the safety and timing of administration of live vaccines. The need for revaccination and the timing of other vaccines in CAR-T recipients remains poorly defined. The current literature is limited and mostly derived from CD19-targeted treatments and is emerging from B-cell maturation antigen CAR-T–treated individuals. There is considerable heterogeneity in vaccine responses which is primarily influenced by the therapeutic target antigen construct and the stage of differentiation at which it is expressed on the B cells. In addition to host-related factors (advanced age, prior lines of therapy), prolonged treatment-related B-cell aplasia, cytopenia, and hypogammaglobulinemia lead to diminished vaccine humoral responses.
接受B细胞耗竭疗法和长期维持治疗的患者
Patients Receiving B-Cell–Depleting Therapies and Those on Chronic Maintenance Treatment
文献回顾与分析
Literature Review and Analysis
观察到某些B细胞恶性肿瘤患者,特别是接受抗CD20抗体治疗的患者,在治疗后的前6-12个月内新冠感染相关的结果较差,且无法产生对新冠疫苗有效的体液免疫反应。37,41,76,170 然而,相当比例的患者观察到了细胞免疫反应。应考虑在完成B细胞耗竭治疗后至少6-12个月进行重新接种新冠疫苗。
It was observed that patients with certain B-cell malignancies, especially those receiving anti-CD20 antibodies, had worse COVID-19–related outcomes and were unable to mount an effective humoral response to the COVID-19 vaccines in the first 6-12 months after treatment.37,41,76,170 However, cellular immune responses were observed in a substantial proportion of patients. COVID-19 revaccination should be considered at least 6-12 months after completion of B-cell–depleting treatments.
临床解读
Clinical Interpretation
理解B细胞耗竭治疗后B细胞恢复和对新冠疫苗的血清学反应动态对于确定针对新冠疫苗接种的最佳时机至关重要。需要考虑的是,由于包括之前治疗的数量和类型、肿瘤组织学以及合并症在内的诸多因素,B细胞恢复可能会延迟。经历反复抗CD20治疗的患者可能会随着每次暴露逐渐减弱B细胞重建。尽管体液免疫反应可能减弱,但疫苗接种仍然强烈推荐,因为细胞免疫反应至少部分保持完好。患者应接种季节性流感疫苗。171 这些疫苗可以在慢性抗CD20定向治疗的最新治疗剂量后4周接种。对于其他非季节性的免疫接种,理想地疫苗接种时机为在开始抗CD20治疗前2-4周或延迟到完成治疗后6-12个月接种,但RZV可以在最近一次B细胞耗竭治疗后1个月接种。102,103,172 B细胞重建的血清学评估可能有助于确定最佳疫苗接种时机。
Understanding the dynamics of B-cell recovery and serological responses to COVID-19 vaccination following B cell-depleting therapy is important in determining the optimal timing of vaccination against COVID-19 infection. It is important to consider that B-cell recovery may be delayed for a variety of reasons including number and type of prior treatments, tumor histology, and comorbidities. Patients undergoing repetitive exposure to anti-CD20 therapies may have progressively attenuated B-cell reconstitution with each successive exposure. Vaccination is still strongly recommended as cellular immune responses appear to remain at least partly intact. Patients should receive seasonal influenza vaccine despite attenuated responses.171 These vaccines can be timed 4 weeks from the most recent treatment dose for patients on chronic anti–CD20-directed therapy. For other nonseasonal immunizations, vaccines ideally should be given 2-4 weeks before commencing anti-CD20 therapy or delayed until 6-12 months after completion, except for RZV, which can be given 1 month after the most recent dose of B-cell–depleting therapy.102,103,172 Serological assessment of B-cell reconstitution may help determine the optimal vaccination timing.
对单克隆抗体治疗CD20单独或与其他联合治疗后引起的低丙种球蛋白血症的风险已经相对较好地获得研究;然而,对于可以耗竭B细胞的新型单克隆抗体和治疗策略,如针对CD20和CD3的双特异性抗体,相关研究数据非常有限。173
The risk of hypogammaglobulinemia following individual monoclonal antibodies against CD20, alone or in combination with other therapies, has been relatively well studied; however, there is a paucity of data for newer monoclonal antibodies and strategies that can deplete B cells, such as bispecific antibodies targeting CD20 and CD3.173
包括长期生存者在内的其他未治疗或控制性血液恶性肿瘤患者
Other Patients With Untreated or Controlled Hematologic Malignancies, Including Long-Term Survivors
血液恶性肿瘤的幸存者通常不会定期检测可能影响疫苗反应的持续免疫缺陷,如低丙种球蛋白血症。然而,一部分血液恶性肿瘤患者由于先天免疫缺陷(例如,慢性淋巴细胞性白血病、小淋巴细胞性淋巴瘤、慢性淋巴瘤)或接受过导致短暂或持久性低丙种球蛋白血症的B细胞靶向治疗,应在较低的阈值下接种非活疫苗。
Survivors of hematologic malignancies are not routinely tested for persistent immune defects that can impact vaccine responses such as hypogammaglobulinemia. However, a subset of patients with hematologic malignancies have an inherent immune defect regardless of treatment (eg, CLL, small lymphocytic lymphoma, indolent lymphomas) or have been exposed to B-cell–directed therapies that predispose to transient or persistent hypogammaglobulinemia, and administration of nonlive vaccines should be instituted at a low threshold.
近期关于新冠疫苗的研究表明,与最近接受过治疗的个体相比,治疗前的患者对疫苗的免疫反应更好,尤其是那些接受过B细胞靶向治疗的患者(例如,抗CD20单克隆抗体、BTK抑制剂、B细胞淋巴瘤-2抑制剂)。174 然而,获得最佳疫苗反应大多需要T细胞和B细胞的激活,103 而一部分持续功能性B细胞破坏的患者可能会保留或恢复T细胞功能,从而对疫苗产生细胞免疫反应,并提供一些保护,这也弥补了常规作为预防措施采用IVIG获得的被动免疫。对于静脉注射免疫球蛋白与灭活疫苗同时使用无需担心免疫干扰;因此,可以同时或近似时间内接种疫苗和使用IVIG。
The recent literature surrounding COVID-19 vaccinations demonstrates that the immune response to vaccination in this group of patients is better in treatment-naïve compared to recently treated individuals, especially those who have received B-cell–directed therapies (eg, anti-CD 20 monoclonal antibodies, BTK inhibitors, B-cell lymphoma-2 inhibitor).174 However, T- and B-cell activation is required for most optimal vaccine responses,103 and a subset of patients with persistent functional B-cell disruption may retain or recover T-cell function, allowing cellular immune response to vaccines and providing some protection that complements the passive immunity provided by IVIG, which is routinely used as a prevention measure in many patients. There is no concern for immune interference with inactivated vaccines with administration of IVIG; thus, coadministration or proximate administration of vaccine and IVIG can occur.
接受治疗或未接受治疗的血液恶性肿瘤患者应遵循表2中阐述的疫苗接种方案。提供医疗服务者应在传染病专家的咨询下,根据具体情况处理活疫苗的安全问题。值得注意的是,未经治疗的血液恶性肿瘤患者可能存在T细胞亚群和抗原呈递细胞的微小功能障碍。因此,使用活疫苗进行免疫接种并不安全。
Individuals with treated or untreated hematologic malignancies should follow the vaccination schedule outlined in Table 2. Providers should address the issue of live vaccine safety on a case-by-case basis in consultation with an infectious diseases expert. It is worth noting that untreated patients with hematologic malignancies may have subtle dysfunctions of T-cell subsets and antigen-presenting cells. Therefore, immunization with a live vaccine is unsafe.
临床问题3:对于计划前往美国以外地区旅行的癌症患者,推荐哪些额外的疫苗接种?
Clinical Question 3: What Additional Vaccinations Are Recommended for Adults with Cancer WHO Are Traveling Outside the United States?
文献回顾与分析
Literature Review and Analysis
2013年的IDSA指南指出,根据CDC为免疫健全成人和儿童制定的年度疫苗接种计划,可以向免疫抑制者接种非活疫苗,但通常不应给免疫抑制患者接种活病毒疫苗。5 更新的文献回顾未发现会改变这些建议的出版物。《2024年CDC黄皮书:国际旅行健康信息》为免疫抑制的旅行者提供了更多细节。175 黄皮书指出,HSCT受者理想情况下应在移植后至少延迟2年外出旅行,并指出需要为HSCT受者完成全面的疫苗重新接种。
The 2013 IDSA guideline notes that nonlive vaccines indicated for travel based on the CDC annual schedule for immunocompetent adults and children may be administered to immunosuppressed individuals, and that live agent vaccines generally should not be given to patients who are immunosuppressed.5 An updated literature review did not identify publications that would change these recommendations. The 2024 CDC Yellow Book: Health Information for International Travel provides additional details for immunocompromised travelers.175 The Yellow Book states that HSCT recipients should ideally delay travel for at least 2 years after transplant, noting the need for complete revaccination of HSCT recipients.
临床解读
Clinical Interpretation
根据2024年《CDC黄皮书:国际旅行健康信息》中关于免疫抑制旅行者的内容,旅行疫苗接种通常应在最后一次化疗后至少3个月并且疾病处于缓和期进行。175 对于处于观察阶段的活跃未治疗实体恶性肿瘤患者,在考虑接种活病毒疫苗之前,应与其医疗保健提供者讨论旅行时间和免疫抑制程度。某些化疗和靶向药物的免疫抑制作用较小,包括已证实与免疫检查点抑制剂(ICIs)安全使用;然而,关于活病毒疫苗对其的安全性,临床数据有限。
Per the 2024 CDC Yellow Book: Health Information for International Travel information for immunocompromised travelers, travel vaccinations should in general be delayed until at least 3 months from last chemotherapy exposure and with disease in remission for patients with solid tumors.175 Patients with active, untreated solid malignant neoplasm under observation only, should discuss travel timing and the degree of immunosuppression with their healthcare provider before consideration of receipt of live virus vaccines. Some chemotherapeutic and targeted agents are less immunosuppressive than others, including demonstrated safety with ICIs; however, there are limited clinical data regarding safety with live agent vaccines.
由于免疫原性和安全性问题,MMR、水痘疫苗及其他活病毒疫苗不应在HSCT后至少24个月内接种,且只有在当时被认为免疫能力正常的受者才可接种减毒活疫苗。因此,HSCT受者理想情况下应在移植后至少2年后旅行,以便完成全面的疫苗重新接种。此建议也适用于CAR-T细胞治疗接受者,尽管相关数据较少。对于处于缓解期的其他血液恶性肿瘤患者,应与传染病或旅行医学专家一起,根据具体情况仔细评估活疫苗的安全性。32 在所有旅行情况下,在旅行疫苗接种前,应与患者的肿瘤科和常规健康保健团队讨论旅行时间。此外,还应注意感染的地区季节性(例如,南半球的流感季节)以及可能正在全球发生的疫情。
Due to immunogenicity and safety concerns, MMR, varicella vaccines, and other live virus vaccines should not be administered to HSCT recipients for at least 24 months post-transplant and only if the recipient is at that time judged immunocompetent. For this reason, HSCT recipients ideally should delay travel ≥2 years after transplant to allow for full revaccination. This recommendation also extends to CAR-T-cell recipients, though data are sparse. For other patients with hematologic malignancies in remission, the safety of live vaccines should be carefully assessed on a case-by-case basis in conjunction with an infectious disease or travel medicine expert.32 In all cases of travel, discussion of travel timing with the patient's oncologic and general health care team is indicated before travel vaccination. Additionally, attention should be paid to the regional seasonality of infections (eg, influenza timing in the Southern Hemisphere) and outbreaks that may be occurring globally.
临床问题4:对于与癌症患者同住或密切接触的成年人,有哪些建议接种的疫苗?
Clinical Question 4: What Are Vaccination Recommendations for Household and Close Contacts of Adults with Cancer?
文献回顾与分析
Literature Review and Analysis
2013年的IDSA指南指出,与免疫抑制患者同住的免疫健全个体可以安全接种所有推荐的活疫苗和非活疫苗,但有一些例外和注意事项。5 该指南5 建议免疫抑制患者的家庭接触者不应使用口服脊髓灰质炎减毒活疫苗,也不建议在近期接受HSCT或有GVHD的患者的家庭接触者中使用流感减毒活疫苗。最新的文献回顾未发现改变这些建议的出版物。
The 2013 IDSA guideline states that immunocompetent individuals who live in a household with immunocompromised patients can safely receive all recommended live and nonlive vaccines, with certain exceptions and precautions.5 The IDSA guideline5 recommends against oral polio vaccine for household contacts of immunocompromised patients, and also recommends against live-attenuated influenza vaccine in household contacts of patients who have recently received an HSCT or have GVHD. An updated literature review did not identify publications that would change these recommendations.
临床解读
Clinical Interpretation
所有由美国免疫实施咨询委员会(ACIP)推荐给成人和儿童的疫苗,包括水痘和MMR等活疫苗,都可以安全地为接受癌症治疗患者的家庭接触者接种,唯一的例外是HSCT接受者的密切接触者不应使用活性减毒流感疫苗。此外,密切接触者应保持与季节性疫苗接种的最新状态。
All vaccines recommended by the ACIP for both adults and children, including live vaccines such as varicella and MMR, can be safely administered to household contacts of patients undergoing cancer treatment except for live attenuated influenza vaccine use in close contacts of HSCT recipients Additionally, close contacts should remain up to date with seasonal vaccines.
减毒活疫苗的抗原如果未完全减毒,偶尔可能毒力恢复为野生型或有效地传播给易感接触者。幸运的是,观察到的传播事件导致意外临床后果非常罕见,除了口服脊髓灰质炎减毒活疫苗和天花疫苗,因此这些疫苗不应给予免疫抑制者的家庭成员。176-178 二价口服脊髓灰质炎减毒活疫苗只在少数国家使用,而天花(活痘苗)疫苗仅在特定情况下建议使用。
An incompletely attenuated live vaccine formulation can occasionally revert to wild type or effectively transmit to a susceptible close contact. Observed transmission events with unintended clinical consequences have fortunately been rare, except for oral poliovirus and smallpox vaccines, which should not be given to family members of immunocompromised persons.176-178 The bivalent oral poliovirus vaccine is only used in a few countries, and the smallpox (live vaccinia) vaccine is indicated for select situations.
关于家庭接触者使用活疫苗的具体考虑:
Specific considerations regarding live vaccine use in household contacts:
·常规儿童和成人免疫的活疫苗:接种通过鼻腔给药的流感减毒活疫苗后,可从鼻拭子中回收到活病毒。179,180 尚未有被证实的流感减毒活疫苗将病毒传播给严重免疫抑制接触者所致的病例。证据表明,对于癌症患者的家庭成员和其他接触者,如果他们的免疫抑制程度为轻到中等,流感减毒活疫苗通常是安全的。接受HSCT的患者的接触者应优先接种灭活疫苗。MMR和水痘疫苗都可以安全地给予密切接触者。家庭成员使用MMR疫苗时,并未发现与疫苗株传播给免疫抑制宿主相关病例。已在发表的文献中描述了11例水痘(vOka)疫苗株传播案例,但没有发生在免疫抑制患者中。181 由于疫苗株可能在极度免疫抑制患者中引起严重甚至致命的水痘,182-186 建议避免与接种水痘疫苗引起的皮疹的人密切接触。水痘疫苗受种者应覆盖皮疹部位,并避免与免疫抑制的家庭成员进行密切的肌肤接触,直到痊愈。轮状病毒疫苗株在接种第一剂疫苗后能够通过粪便排出,有报道称密切接触者出现相关胃肠炎。187,188 建议保持良好的手卫生习惯并避免在最后一剂疫苗后至多2周内更换尿布,以降低传播风险。
○ Live vaccines for routine childhood and adult immunization of household contacts: Viable virus can be recovered from nasal swabs after intranasally administered influenza vaccine.179,180 No confirmed cases of live attenuated influenza vaccine virus transmission to severely immunocompromised contacts have been described. The evidence indicates that live attenuated influenza vaccine is generally safe for family members and other contacts of patients with cancer who have mild to moderate immunosuppression. Contacts of patients who receive HSCT should preferably receive the inactivated vaccine. MMR and varicella vaccines are both safe to administer to close contacts. Vaccine strain transmission to immunocompromised hosts is not associated with MMR use in family members. Eleven cases of varicella (vOka) vaccine strain transmission are described in the published literature, but none occurred in immunocompromised patients.181 Because vaccine strain can cause severe and fatal varicella in profoundly immunocompromised patients,182-186 precautions are advised to avoid close contact with a person with vaccine-induced rash. The rash site should be covered, and intimate skin-to-skin contact should be avoided with the immunocompromised household member until healed. Vaccine strain rotavirus is shed in the stool after the first vaccine dose, with reports of gastroenteritis in close contacts.187,188 Practice of good hand hygiene and avoidance of diaper changes for up to 2 weeks after the last vaccine dose is recommended to reduce the risk of transmission.
·家庭接触者旅行者的活疫苗:MMR和黄热病疫苗的使用是安全的。 口服伤寒疫苗(口服Ty21a减毒活疫苗)的使用也是可以接受的,尽管在接种后在粪便中检测到活菌体,但没有发现进一步的传播。2016年,口服霍乱疫苗在美国获得旅行者使用许可。大约11%的健康疫苗接受者会在接种后最多7天内在粪便中排出疫苗菌株。尽管传播能力总体看来较低,且不超过7天,但现有数据有限。因此,不应给免疫抑制患者的家庭接触者接种口服霍乱疫苗。
○ Live vaccines for household contact travelers: The use of MMR and yellow fever vaccines is safe. Oral typhoid vaccine (Live Oral Ty21a) use is also acceptable, although live organisms have been detected in the stool postimmunization, albeit without onward transmission. In 2016, the oral cholera vaccine was licensed for US travelers. Approximately 11% of healthy vaccine recipients excrete the vaccine strain bacteria in their stool for up to 7 days. Although the transmission potential appears to be low overall, available data are limited and do not extend beyond 7 days. Therefore, the oral cholera vaccine should not be given to household contacts of immunocompromised patients.
·其他基于风险考虑的活疫苗:第二代天花疫苗(ACAM2000)包含在细胞中培养的痘苗病毒,获批用于特定的军事人员、实验室和研究工作者,以及在美国预防猴痘。ACAM 2000接种后意外传播痘苗病毒的风险与第一代天花疫苗(Dryvax)相似,估计约为每10万次接种中有5.4起事件。189,190 因此,不应在免疫抑制患者的家庭成员中使用ACAM-2000。相较下,一种基于减毒活的改良痘苗安卡拉疫苗(Jynneos)被批准用于预防猴痘,该疫苗对免疫抑制患者的家庭接触者是安全的。191 最后,一种皮下注射的登革热减毒活疫苗最近在美国获得批准。尽管这种疫苗不能给癌症患者接种,符合条件的儿童居住在免疫抑制患者的家庭中可以接种这种疫苗。192
○ Live vaccines for other risk-based indications: A second-generation smallpox vaccine containing the vaccinia virus grown in cell culture (ACAM2000), is used for certain military personnel, laboratory and research workers, and for monkeypox prevention in the United States. The risk of unintentional vaccinia transmission after ACAM 2000 is similar to the first-generation smallpox vaccine (Dryvax) and is estimated at around 5.4 events per 100,000 vaccinations.189,190 Therefore, ACAM-2000 should not be used in household members of immunocompromised patients. In contrast, a live replication-deficient modified vaccinia Ankara vaccine (Jynneos) to prevent monkeypox is safe for household contacts of immunocompromised patients.191 Finally, a subcutaneously administered live dengue vaccine was recently licensed in the United States. Although this vaccine cannot be given to patients with cancer, eligible children who reside in the household of an immunocompromised person can receive this vaccine.192
讨论
Discussion
感染是癌症确诊后第一年内非癌症相关死亡的第二大常见原因,其中大多数死亡归因于流感和肺炎,这些死亡可以通过接种疫苗进行预防。9,193 尽管癌症患者对流感和肺炎球菌疫苗的免疫反应较低,但证据支持疫苗接种在减少感染严重程度和相关住院方面的安全性和获益。
Infections are the second most common cause of non–cancer-related mortality within the first year after a cancer diagnosis, with most of these deaths attributed to influenza and pneumonia, deaths that can be prevented through immunization.9,193 While patients with cancer have lower immune responses to influenza and pneumococcal vaccines, evidence supports the safety and benefits of vaccinations in reducing the severity of infections and associated hospitalizations.
此外,如mRNA新冠疫苗和重组带状疱疹疫苗等新疫苗在许多癌症患者中的有效性只略低于普通人群。新冠疫苗的经验强调了细胞介导免疫在预防重症中的重要作用。这突显了许多癌症患者能够从疫苗接种中受益,包括那些抗体产生减弱的患者。
Furthermore, newer vaccines, such as COVID-19 mRNA vaccines and the RZV, demonstrate only slightly lower efficacy in many patients with cancer when compared with the general population. The COVID-19 vaccine experience emphasizes the important role of cell-mediated immunity in preventing severe disease. It underscores that many patients with cancer benefit from vaccination, including those with blunted antibody production.
癌症患者对疫苗反应减弱和疫苗保护不足的普遍看法应该演变为强调预防严重疾病的重要性,以及通过疫苗接种减少感染相关并发症在优化癌症相关结果方面的关键作用。
The common perception of weakened vaccine responses and inadequate protection of vaccines in patients with cancer should evolve to emphasize the importance of preventing severe disease and the critical role of vaccines in enhancing cancer-related outcomes by reducing infection-related complications.
被诊断为癌症可能令人难以承受,且疫苗接种在治疗计划中可能不是首要优先事项。然而,许多研究一致突出显示,在开始癌症治疗前接种疫苗能够提供最佳保护,强调了早期接种的必要性。
A cancer diagnosis can be overwhelming, and vaccination may not be an immediate priority in the treatment plan. However, numerous studies consistently highlight the best protection when vaccines are administered before starting cancer treatment, emphasizing the need for early vaccination.
为实现这一目标,优化疫苗接种状态应被视为癌症患者护理中的一个关键元素,通过多学科方法和专用资源实现,遵循成人免疫实践建议。14 实施中应考虑包括以下几点:
To achieve this goal, optimizing vaccination status should be considered a key element in the care of patients with cancer, through multidisciplinary approaches and dedicated resources, following Standards for Adult Immunization Practices.14 Implementation considerations include the following:
1.在患者首诊时记录疫苗接种状态,有助于识别需要更新疫苗接种或需要季节性疫苗接种的患者。
1. Documentation of vaccination status at the time of the first patient visit will help identify patients who need their vaccinations brought up to date or for whom seasonal vaccinations are required.
2.HCST、CAR-T治疗或B细胞耗竭治疗后的重新接种与免疫重建的预期时间的一致。
2. Alignment of revaccination after HCST, CAR-T therapy, or B-cell–depleting treatments with the expected time of immune reconstitution.
3.与初级保健提供者、药剂师和护理同事的积极合作,收集并反馈疫苗接种数据。
3. Active partnerships with patients' primary care providers, pharmacists, and nursing colleagues to collect and respond to vaccination data.
4.提供服务者的支持和患者教育对于克服癌症治疗患者在疫苗使用方面的犹豫和常见误解至关重要。
4. Provider endorsement and patient education are essential to overcome vaccine hesitancy and common misconceptions related to vaccine use for patients undergoing cancer treatment.
5.强调为家庭接触者和护理人员接种疫苗,以保护患者。
5. Emphasis on vaccination of household contacts and caregivers to protect patients.
当前差距与未来方向
Current Gaps and Future Directions
对癌症患者和长期癌症幸存者的免疫抑制进行持续研究对于深入理解免疫系统如何处理疫苗和疾病是至关重要的。这些研究能极大提升对患者护理的理解,并超出日常临床应用。疫苗可预防疾病的加强接种不仅有利于患者个体,对于保护社区健康和提升癌症患者护理质量非常重要。以下是一些需要持续研究和额外努力的关键领域:
Continuous research into the mechanisms and remedies for acute and chronic immunocompromise in patients with cancer and long-term cancer survivors and into how the immune system processes and responds to vaccine and disease exposure will advance knowledge well beyond the direct implications for patient care. Enhancing vaccine uptake against preventable illnesses will help the community and improve the quality of care for patients with cancer. Unmet needs that require continuous research and other dedicated efforts include the following:
·疫苗试验中纳入癌症患者: 必须让不同免疫状态的癌症患者参与疫苗研究。为了确保有意义的代表性,应积极招募正在接受治疗或近期接受过治疗的癌症患者参与疫苗研究。
• Participation of patients with cancer with varied types of immunocompromise in vaccine trials is imperative. To ensure meaningful representation, actively or recently treated patients with cancer should be recruited actively to vaccine trials. Where vaccine trials for only patients with cancer are not feasible, pre-existing cancer should not preclude eligibility and inclusion of cohorts of patients receiving anticancer treatment(s) should be incorporated prospectively.
寻求更具免疫原性的疫苗: 寻找更有效的疫苗及优化接受新型治疗的癌症患者的疫苗接种策略是非常关键的。了解CAR-T治疗、新型B细胞靶向治疗和针对免疫调节剂的双特异性抗体后的疫苗反应至关重要。
• The quest for more immunogenic vaccines and research to optimize vaccination approaches in patients receiving novel therapies is vital. There is a critical need to expand the understanding of vaccine responses after CAR-T, newer B-cell–directed therapies, and bispecific antibodies that target immune modulators as one epitope. These populations might have unique needs because of the underlying effect of disease and previous therapy on immune function, the potential for loss of immunity to childhood immunizations, and impairment of future vaccine responses.
·帮助医疗机构承诺并维持癌症患者的疫苗接种最佳实践: 通过多学科团队方法,建立并持续推广癌症患者疫苗接种的最佳实践是必要的。应利用数据共享,标准化癌症患者疫苗接种状态和需求的记录过程。
• Mechanisms to help successful institutional commitment to integrate and sustain immunization best practices for patients with cancer through multidisciplinary team-based approaches, protocol-based vaccination standing orders, and leveraging data sharing to implement standardized processes for finding and recording a patient's vaccination status and needs.
·基于证据的决策工具,强调预防护理: 提供教育资源和培训,解答有关疫苗接种的常见问题和误解。
• Evidence-based decision-making tools emphasizing preventative care through immunization. Provide educational resources and training to address commonly asked questions and misperceptions.
·应对癌症治疗期间疫苗犹豫的挑战和因素的策略: 制定策略来解决癌症治疗期间疫苗犹豫的问题是必要的,这将有助于提高这一脆弱群体的疫苗接种率。
• Strategies for addressing the unique challenges and factors contributing to vaccine hesitancy during cancer treatment.
基于团队化规划改善疫苗接种率
Provider Team-based Protocols to Improve Vaccine Uptake
在血液学和肿瘤学以及其他专科设置中,采用疫苗接种规划已被证明可以提高流感和肺炎球菌疫苗的接种率。Wong等194实施了一项疫苗接种规划作为质量倡议,使疫苗接种率从不足70%提高到89%甚至更高。Rodriguez等195描述的护士主导的规划使流感疫苗接种数量增加了97%,肺炎球菌疫苗接种数量增加了684%。通过协作的多学科方法开发的有效疫苗接种规划最好整合到诊所的工作流程中。这种方法的一个成功例子始于患者抵达诊所时。电子病历中生成的电子提醒或最佳实践警报将促使诊所或护理人员对患者进行资格筛查。如适用,护士将激活常规指令集或启动疫苗接种的草案订单,待提供者签名并激活后,患者在离开医疗设施前接种相应的疫苗。194-197 护理评估和参与为患者和护理者提供了教育、支持和加强癌症治疗期间疫苗接种的安全性和有效性的机会。
Implementation of a vaccination protocol has been shown to improve influenza and pneumococcal vaccination rates in the hematology and oncology and other specialty settings. Wong et al194 implemented a vaccination protocol as a quality initiative, increasing vaccination rates from below 70% to 89% and higher. A nurse-led protocol described by Rodriguez et al195 increased the number of influenza vaccinations by 97% and the number of pneumococcal vaccinations by 684%. Developed through a collaborative multidisciplinary approach, effective vaccination protocols are best integrated into the clinic's workflow processes. A successful example of this approach begins on the patient's arrival at the clinic. An electronic reminder or best practice alert generated in the electronic medical record will prompt patient screening for eligibility by the clinic or nursing staff. If applicable, the nurse activates a standing order set or initiates draft orders for vaccination to be signed and activated by the provider, and the patient receives the applicable vaccine(s) before leaving the health care facility.194-197 Nursing assessment and engagement provide the opportunity for patient and caregiver education, support, and reinforcement of the safety and efficacy of vaccinations during cancer treatment.
致谢 Acknowledgment
专家小组感谢 Eric Roeland 博士、Christina Annunziata 博士以及循证医学委员会对本指南的深思熟虑的审查和富有洞察力的评论。
The Expert Panel would like to thank Dr Eric Roeland, Dr Christina Annunziata, and the Evidence-Based Medicine Committee for their thoughtful reviews and insightful comments on this guideline.
循证医学委员会批准日期:
Evidence-Based Medicine Committee approval:
2023年12月14日
December 14, 2023
补充数据、编者按等信息,可向我发送PDF版需求,请明确告知姓名、工作单位、邮箱地址,如介意信息泄露,可点击【阅读原文】看原始版本,感谢您的理解。
愿

=丸=
免责声明:本文为个人兴趣创作,仅为让更多普通人对疫苗有更加清晰的认识,内容观点不代表任何组织、单位、机构,未接受任何形式赞助,所有配图均来自网络公开平台,如果内容有误,大家多做自我批评(不是)。




 扫码下载APP
扫码下载APP

 科普中国APP
科普中国APP
 科普中国
科普中国
 科普中国
科普中国